Process: Phosphomolybdate-Mediated 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wacker Oxidation ~Anti-Markovnikov~
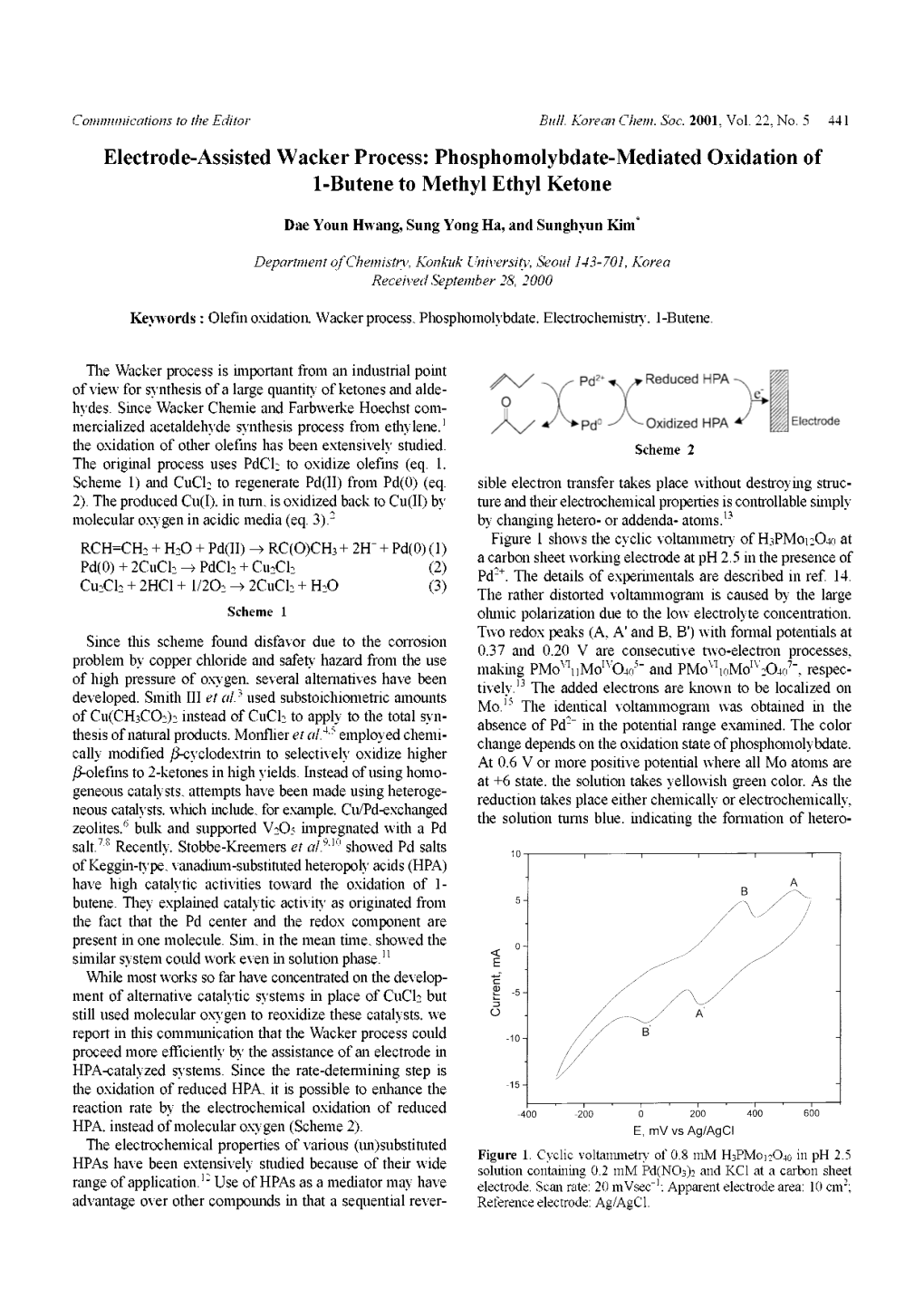
Anti-Markovnikov Olefin Functionalization ~Prof. Robert H. Grubbs’ Work~ 4th Literature Seminar July 5, 2014 Soichi Ito (D1) Contents 1. Introduction • Flow of Prof. Grubbs’ Research • Markovnikov’s Rule • Wacker Oxidation 2. Grubbs’ Work • Substrate-Controlled Wacker Oxidation • Catalyst-Controlled Wacker-Type Oxidation 2 Introduction ~Flow of Research~ Olefin Metathesis Anti-Markovnikov Wacker Oxidation of Terminal Olefin Substrate-Controlled Wacker Oxidation of Internal Olefin Z-Selective Metathesis Hydration Ethenolysis + Reduction Hydroamination Z-Selective Ethenolysis Catalyst-Controlled Decarbonylative Dehydration Hydrophosphonation Production of Terminal Olefin Functionalization of Terminal Olefin 3 Introduction ~Markovnikov’s Rule~ Two-Step Two-Step (+1C) 4 Robert H. Grubbs et al. Science, 2011, 333, 1609. Anti-Markovnikov Hydration of Olefins • One-Step William C. Trogler et al. Science 1986, 233, 1069. This work was difficult to reproduce. Inorg. Chem. 1988, 27, 3151. • One-Step with Activated Olefins Robert G. Bergman and F. Dean Toste et al. J. Am. Chem. Soc. 2003, 125, 8696. Ben L. Feringa and Gerard Roelfes et al. Nat. Chem. 2010, 2, 991. • Three-Step 5 Shannon S. Stahl et al. J. Am. Chem. Soc. 2010, 132, 15116. Anti-Markovnikov Wacker Oxidation / Reduction Strategy Oxidation cycle must be compatible with the reduction cycle. aldehyde-selective Wacker Oxidation 6 Robert H. Grubbs et al. Science, 2011, 333, 1609. Introduction ~Wacker-Tsuji Oxidation~ • 1894 F. C. Phillips reported stoichiometric reaction. • 1959 J. Smidt et al. reported the Wacker process. (oxidation of ethylene to acetaldehyde) Investigations for convenient laboratory methods • 1976 J. Tsuji et al. reported PdCl2, CuCl / DMF, H2O method. “Terminal alkenes may be viewed as masked ketones.” 7 Jacques Muzart Tetrahedron 2007, 63, 7505. -

New Reactions of Cyclic Oxygen, Nitrogen and Sulfur Acetal Derivatives
New Reactions of Cyclic Oxygen, Nitrogen and Sulfur Acetal Derivatives Samuel Edward Mann Supervisor Dr. Tom Sheppard A thesis submitted to University College London in partial fulfilment of the requirements for the degree of Doctor of Philosophy Declaration I, Samuel Edward Mann confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. ………………………… i Abstract Abstract This thesis describes the development of new reactions of cyclic oxygen, nitrogen and sulfur acetal derivatives and their applications in a diverse range of synthetic organic and organometallic chemistry. Detailed herein are advances in three main areas of acetal chemistry, namely: studies towards a new methodology for the synthesis of medium ring heterocycles; the use of thioacetals as directing groups for the palladium-mediated oxidation of olefins; and multi-component reactions for the synthesis of drug-like heterocyclic compounds. A brief overview of the chemistry of cyclic acetal derivatives is given in the first chapter, followed by a chapter on each of the three areas investigated. Relevant introductory literature is reviewed at the beginning of each chapter. Firstly, the ring expansion chemistry of unsaturated cyclic oxygen, nitrogen and sulfur acetal derivatives was explored for the development of a new methodology for the synthesis of medium ring heterocycles. This methodology has thus far proved unsuccessful in the synthesis of medium rings, although several interesting and unusual transformations were observed, such as the unexpected formation of an intriguing bicyclic enaminium salt. The use of thioacetals as directing groups for the palladium-mediated oxidation of terminal olefins was also explored, leading to the evolution of a new methodology for the catalytic, regioselective formation of either vinyl or allylic acetates. -

Organic Seminar Abstracts
Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/organicsemin1971752univ / a ORGANIC SEMINAR ABSTRACTS 1971-1972 Semester II School of Chemical Sciences Department of Chemistry- University of Illinois Urbana, Illinois 3 SEMINAR TOPICS II Semester 1971-72 Reactions of Alkyl Ethers Involving n- Complexes on a Reaction Pathway 125 Jerome T. Adams New Syntheses of Helicenes 127 Alan Morrice Recent Advances in Drug Detection and Analysis I36 Ronald J. Convers The Structure of Carbonium Ions with Multiple Neighboring Groups 138 William J. Work Recent Reactions of the DMSO-DCC Reagent ll+O James A. Franz Nucleophilic Acylation 1^2 Janet Ollinger The Chemistry of Camptothecin lU^ Dale Pigott Stereoselective Syntheses and Absolute Configuration of Cecropia Juvenile Horomone 1U6 John C. Greenfield Uleine and Related Aspidosperma Alkaloids 155 Glen Tolman Strategies in Oligonucleotide Synthesis 162 Graham Walker Stable Carbocations: C-13 Nuclear Magnetic Resonance Studies 16U Moses W. McMillan Organic Chlorosulfinates 166 Steven W. Moje Recent Advances in the Chemistry of Penicillins and Cephalosporins 168 Ronald Stroshane Cerium (iv) Oxidations 175 William C. Christopfel A New Total Synthesis of Vitamin D 18 William Graham Ketone Transpositions 190 Ann L. Crumrine - 125 - REACTIONS OF ALKYL ETHERS INVOLVING n-COMPLEXES ON A REACTION PATHWAY Reported by Jerome T. Adams February 2k 1972 The n-complex (l) has been described as an intermediate on the reaction pathway for electrophilic aromatic substitution and acid catalyzed rearrange- ment of alkyl aryl ethers along with sigma (2) and pi (3) type intermediates. 1 ' 2 +xR Physical evidence for the existence of n-complexes of alkyl aryl ethers was found in the observation of methyl phenyl ononium ions by nmr 3 and ir observation of n-complexes of anisole with phenol. -

Manipulating Selectivity and Reactivity in Palladium-Catalyzed Oxidation Reactions
Manipulating Selectivity and Reactivity in Palladium-Catalyzed Oxidation Reactions Thesis by Zachary Kimble Wickens In Partial Fulfillment of the Requirements for the degree of Doctor of Philosophy CALIFORNIA INSTITUTE OF TECHNOLOGY Pasadena, California 2015 (Defended June 2nd, 2015) © 2015 Zachary Kimble Wickens All Rights Reserved 1 Dedicated to my parents for never pushing me to become a scientist 2 Acknowledgments The work I have the pleasure of sharing with you in this thesis would have been completely impossible without the help (both emotional and intellectual) of a tremendously large number of people. First, I want to thank my advisor, Bob Grubbs. I truly could not have asked for a more incredible thesis advisor. Since the beginning of my time at Caltech, you have subtly guided me towards the water but never forced me to drink. You have given me independence in choosing which projects to pursue and the directions these projects go, but always provided suggestions and hints along the way that have kept me on track. I know you will continue to provide great advice and strong support even long after I've left Caltech. Thanks for being my invisible safety net over the last five years. I would have never even considered pursuing a Ph.D in chemistry in the first place if it weren't for the fantastic chemistry faculty at Macalester College. Rebecca Hoye, Ronald Brisbois, Paul Fischer, Keith Kuwata, Thomas Varberg and Katherine Splan, you were all tremendously influential in my life. You taught me how to see the exciting puzzles that are scattered throughout the field of chemistry. -

Novel Co-Mo/MCM-41 Catalysts for Deep Hydrodesulfurization of Jet Fuel
Elucidating the mechanism of heterogeneous Wacker oxidation over response from Pd speciation (Figure 1) demonstrating for the first time that oxygen is activated Pd-Cu-exchanged zeolite Y via transient multi-edge XAS on copper in the heterogeneous system, which is similar to the homogeneous system. Increasing the O partial pressure led to a quick reoxidation of Cu(I) with a concomitant rapid 2 increase in CO formation while that of acetaldehyde proceeded more slowly. These results Jerick Imbao1,2, Jeroen van Bokhoven1,2*, Adam Clark1 and Maarten Nachtegaal1* 2 indicate that copper has more than one role as it does not only act as a co-catalyst for Pd(0) 1Paul Scherrer Institute, Villigen PSI (Switzerland) regeneration, but is also involved in the formation of CO byproduct. Without detecting the 2ETH Zurich, Zurich (Switzerland) 2 transient presence of Cu(0) and Pd(I), our results suggest that two one-electron transfers from *[email protected], [email protected] two Cu(II) ions to reoxidize Pd(0) to Pd(II) is at work in this heterogeneous Wacker catalyst. Introduction Low O (half order) High O (zero order) The homogeneous ethylene oxidation, called the Wacker process, is one of the most 1.00 2 1.00 2 efficient synthetic methods for manufacturing aldehydes. This process suffers from the difficulty in the separation of the products from the catalyst and from the high corrosivity and 0.75 0.75 formation of chlorinated byproducts associated with the excess use of HCl [1]. To overcome Cu(II) Cu(II) these problems many studies have been undertaken to design chloride-free heterogeneous 0.50 0.50 Cu(I) Cu(I) Wacker catalyst systems. -

Improved Catalyst System for the Wacker Process
potential user’s site in container drums. The salts during the methanol drying technique, results from the trial, which should last for at then fast economic pay-back times can be anti- least 1000 hours of continuous operation, will cipated and a remarkably cheap hydrogen allow the viability of the process to be proven source deployed. J.E.P. at small cost, before a full size plant is commis- References sioned. I J. E. Philpott, Platinum Metals Rev., 1976, to, Where a trial is successful and when the (4), I 10; and references therein operating variables are established, including, 2 M. J. C(ole), Platinum Metals Rev., 1981,25, (I), I2 for example, the regularity of water rinsing 3 J. E. Philpott, Platinum Metals Rev., 1985, 29, necessary to prevent the build up of ammonium (I), 12; and references therein Improved Catalyst System for the Wacker Process The Wacker process for the palladium plete oxidation of ethylene to carbon dioxide catalysed conversion of ethylene to and water is avoided. acetaldehyde is one of the oldest industrial The authors have combined a number of con- homogeneous catalyst systems. The original cepts which have been available for some time, process was plagued by problems of corrosion and appear to have developed a system which within the reactor system, and has been replac- avoids most of the pitfalls associated with other ed by a heterogeneous catalyst system which catalyst systems for the Wacker process. If it suffers, however, from the problem of poor can be established that the catalyst maintains its catalyst utilisation. -

The Oxidation of Terminal Olefins to Methyl Ketones by Jones Reagent Is Catalyzed, by Mercury(Il)
[Reprinted from the .Journalof Organic Chemistry'.{0. i]r77 ( 1975).l Copvright 19?5by the American Chemical Societvand reprinted bv permissionof the copyright owner. The Oxidation of Terminal Olefins to Methyl Ketones by Jones Reagent Is Catalyzed,by Mercury(Il)t Harold R. Rogers, Joseph X. McDermott,2 and George M. Whitesides* Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 ReceiuedJune 11. 1975 The oxidation of terminal olefins by Jones reagent in the presence of a catalytic quantity of mercury(II) affords good yields (>lO"t") of the corresponding methyl ketones. Similar oxidations of 1,2-disubstituted olefins gives fair (20-70%) yields; in the caseof unsymmetrically substituted olefins, mixtures of ketones are produced. The Wacker process for oxidation of olefins to ketones products) different from those of the Wacker oxidation. has three mechanistically distinct parts:3 first, activation of Mercury(Il) is an obvious candidate for the catalyst for the olefinic double bond toward nucleophilic attack by new oxidation reactions: it resembles palladium(Il) in its coordination with Pd(II) and addition of a hydroxide moi- ability to activate olefins for nucleophilic attack,a but dif- ety to this electrophilic double bond; second, conversion of fers in that decomposition of the oxymercuration products the resulting 2-hydroxyethylpalladium(Il) compound to normally generates cations by loss of mercury(0) rather ketone and a (formally) Pd(O) atom by a series of palladi- than olefins by loss of mercury hydride.s Unfortunately, um(II) hydride addition-eliminations involving vinylic al- neither we nor others6 have been able to discover a satisfac- cohol intermediates; third, reoxidation of the palladium(0) tory solution to the principal problem in developing a mer- to palladium(Il) by copper(Il). -

Transition Metal Catalysed Reactions for the Synthesis of Heteroaromatic Compounds
Transition Metal Catalysed Reactions for the Synthesis of Heteroaromatic Compounds Stephen Christopher Pelly A thesis submitted to the Faculty of Science, University of the Witwatersrand, Johannesburg In fulfilment of the requirements for the Degree of Doctor of Philosophy January 2007 DECLARATION I declare that the work presented in this thesis was carried out exclusively by myself under the supervision of Professor C.B. de Koning. It is being submitted for the degree of Doctor of Philosophy in the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination in any other University. _________________ 27th day of January, 2007 i ABSTRACT The carbazole and 2-isopropenyl-2,3-dihydrobenzofuran structures are widely found in many naturally occurring compounds. For example, the naturally occurring anti-cancer compound, rebeccamycin, contains an indolocarbazole core. Rotenone, which contains an (R)-2-isopropenyl-2,3-dihydrobenzofuran moiety, is widely used today as an effective naturally occurring pesticide. In the carbazole section of this thesis, the synthesis of the naturally occurring furanocarbazole, furostifoline is described. As key steps in this sequence, a Suzuki coupling reaction is utilised to couple appropriately functionalised indole and furan moieties. After further functional group transformations, a metathesis reaction is employed to construct the carbazole system, leading to furostifoline. The synthesis of the unnatural thio-analogue of furostifoline was similarly conducted and is described. Finally, in a somewhat different approach, the synthesis of the indolocarbazole core is described, utilising a Madelung approach initially to form the bis-indole system, 2,2’-biindolyl. After several functional group transformations, a metathesis reaction was once again successfully employed to form the carbazole system, thereby synthesising di(tert-butyl) indolo[2,3-a]carbazole-11,12-dicarboxylate. -

Copyrighted Material
JWST960-SUBIND JWST960-Smith October 25, 2019 9:5 Printer Name: Trim: 254mm × 178mm SUBJECT INDEX The vast use of transition metal catalysts in organic chemistry makes the citation of every individual metal impractical, so there are limited citations of individual metals. Palladium is one exception where individual citations are common, in keeping with the widespread use of that metal. However, in most cases, the term metal catalyst, or catalyst, metal is used as a heading, usually representing transition metals. A-SE2 mechanism 893 and the steering wheel model acceleration of Diels-Alder reactions 487 151–152 reactions, high pressure A1 mechanism, acetal hydrolysis and universal NMR database 1038 487 155 hydrogen-bonding 1038 A1,3-strain 196 Cahn-Ingold-Prelog system hydrophobic effect 1038 A2 mechanism, acetal hydrolysis 149–152 in water 1038 487 determination 152 ionic liquids 1038 ab initio calculations 36 D/L nomenclature 149 micellular effects 1038 and acidity 346 Kishi’s NMR method 155 microwave irradiation 1038 and antiaromaticity 71 sequence rules 149–152 phosphate 1039 and nonclassical carbocations absolute hardness 64, 359, 361 solid state 1038 427 table 361 ultracentrifuge 1038 norbornyl carbocation 436 absorbents, chiral 168 ultrasound 1038 ab initio studies 248 absorption, and conjugation 317 zeolites 1038 1,2-alkyl shifts in alkyne anions differential, and diastereomers acceleration, Petasis reaction 1349 168 1202 and cubyl carbocation 413 differential, and resolution 169 acenaphthylene, reaction with and SN2 408–409 abstraction, -

Development of Palladium Catalyzed Alkene Difunctionalization on Vinyl-Quinoline Type Substrate and Isolation of Pd-Alkyl Intermediate
University of Denver Digital Commons @ DU Electronic Theses and Dissertations Graduate Studies 1-1-2016 Development of Palladium Catalyzed Alkene Difunctionalization on Vinyl-Quinoline Type Substrate and Isolation of Pd-Alkyl Intermediate Lusha Xu University of Denver Follow this and additional works at: https://digitalcommons.du.edu/etd Part of the Chemistry Commons Recommended Citation Xu, Lusha, "Development of Palladium Catalyzed Alkene Difunctionalization on Vinyl-Quinoline Type Substrate and Isolation of Pd-Alkyl Intermediate" (2016). Electronic Theses and Dissertations. 1185. https://digitalcommons.du.edu/etd/1185 This Thesis is brought to you for free and open access by the Graduate Studies at Digital Commons @ DU. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ DU. For more information, please contact [email protected],[email protected]. Development of Palladium-Catalyzed Alkene Difunctionalization on Vinyl-Quinoline Type Substrate and Isolation of Pd-Alkyl Intermediate A Thesis Presented to the Faculty of Natural Sciences and Mathematics University of Denver In Partial Fulfillment of the Requirements for the Degree Master of Science by Lusha Xu August 2016 Advisor: Brian W. Michel ©Copyright by Lusha Xu 2016 All Rights Reserved Author: Lusha Xu Title: Development of Palladium-Catalyzed Alkene Difunctionalization on Vinyl-Quinoline Type Substrate and Isolation of Pd-Alkyl Intermediate Advisor: Brian W. Michel Degree Date: August 2016 ABSTRACT The synthesis of quinoline derivatives is a continuing issue facing organic chemists in both academia and industry. To address this problem, palladium-catalyzed alkene difunctionalization was developed to be a powerful and straightforward strategy of synthetic transformations for adding diversity to organic molecules. -

1 the Applications of Water As Reagents in Organic Synthesis
1 1 The Applications of Water as Reagents in Organic Synthesis Zhengkai Chen and Hongjun Ren Zhejiang Sci-Tech University, Department of Chemistry, Hangzhou 310018, PR China 1.1 Introduction Water due to its low cost, easy availability, nontoxic and nonflammable properties has been considered one of the most ideal and promising solvents in organic synthesis from the green and sustainable point of view. Furthermore, with regard to enormous enzyme-catalyzed biosynthesis in nature, water serves as a favorable medium for the versatile synthesis of a variety of complicated molecules and compounds. Over the past decades, considerable efforts had been devoted into the organic reactions by using water as solvent from economy and environment perspectives [1]. Even more, the proposed concept of “in-water” and “on-water” further stimulated the booming development of the utilization of water as solvent for organic synthesis [1e, 2]. Therefore, in recent years, more and more general organic reactions were successfully exploited to perform in water instead of organic solvents to achieve sustainable and environmental benefits. From a different perspective, the water itself could also be applied as a useful reagent to participate in the reaction through incorporating a hydrogen or oxygen atom or hydroxyl group into the target product. Generally, water is indispensable for various hydrolysis reactions. As a hydrogen source, water is used to quench numerous susceptible reaction systems by providing active hydrogen. Meanwhile, as a versatile nucleophile, the hydroxyl group could be readily introduced into the specific reaction sites by the employment of water as hydroxyl precursor. The hydroxyl group could also be readily oxidized to carbonyl group during the reaction. -

PART 3 Principles and Applications of Organometallics in Catalysis
PART 3 Principles and Applications of Organometallics in Catalysis 1. Oxidative Addition and Reductive Elimination These processes are of great importance in synthesis and catalysis using organometallics. A ∆ OS=+2 oxidative addition ∆ LnM+AB LnM CN=+2 reductive elimination B ∆ VE=+2 16 VE 18 VE In the OA we break the (2-electron) A—B bond and form two (2-electron) bonds to the metal, i.e. M—A and M—B. Hence, there are some requirements for OA to occur. (i) A vacant coordination site must be available on the metal complex, and (ii) the metal must have an accessible oxidation state 2 units higher. Conversely, for RE to occur, there must be a stable oxidation state 2 units lower than that in the starting complex. The resulting complex will necessarily have a free coordination site which may make it reactive or perhaps unstable. The position of the OA/RE equilibrium is determined by the overall thermodynamics of the system, i.e. the relative stabilities of the two oxidation states, the relative bond strengths of A—B, M—A and M—B, and entropy terms. 2. Insertion and Elimination X Once we have arranged 1,1-migratory insertion X our ligands by addition MAB MA (either oxidative or simple) we can combine B and transform them 18 V.E. 16 V.E. within the coordination sphere by the process of insertion and its reverse X X elimination. The two 1,2-migratory insertion A A main types of insertion M M B are shown here. In B principle, the reactions are 18 V.E.