Leaching of Styrene and Other Aromatic Compounds in Drinking Water from PS Bottles
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Reinforcement of Styrene Butadiene Rubber Employing Poly(Isobornyl Methacrylate) (PIBOMA) As High Tg Thermoplastic Polymer
polymers Article Reinforcement of Styrene Butadiene Rubber Employing Poly(isobornyl methacrylate) (PIBOMA) as High Tg Thermoplastic Polymer Abdullah Gunaydin 1,2, Clément Mugemana 1 , Patrick Grysan 1, Carlos Eloy Federico 1 , Reiner Dieden 1 , Daniel F. Schmidt 1, Stephan Westermann 1, Marc Weydert 3 and Alexander S. Shaplov 1,* 1 Luxembourg Institute of Science and Technology (LIST), 5 Avenue des Hauts-Fourneaux, L-4362 Esch-sur-Alzette, Luxembourg; [email protected] (A.G.); [email protected] (C.M.); [email protected] (P.G.); [email protected] (C.E.F.); [email protected] (R.D.); [email protected] (D.F.S.); [email protected] (S.W.) 2 Department of Physics and Materials Science, University of Luxembourg, 2 Avenue de l’Université, L-4365 Esch-sur-Alzette, Luxembourg 3 Goodyear Innovation Center Luxembourg, L-7750 Colmar-Berg, Luxembourg; [email protected] * Correspondence: [email protected]; Tel.: +352-2758884579 Abstract: A set of poly(isobornyl methacrylate)s (PIBOMA) having molar mass in the range of 26,000–283,000 g mol−1 was prepared either via RAFT process or using free radical polymerization. ◦ These linear polymers demonstrated high glass transition temperatures (Tg up to 201 C) and thermal Citation: Gunaydin, A.; stability (T up to 230 ◦C). They were further applied as reinforcing agents in the preparation of the Mugemana, C.; Grysan, P.; onset Eloy Federico, C.; Dieden, R.; vulcanized rubber compositions based on poly(styrene butadiene rubber) (SBR). The influence of the Schmidt, D.F.; Westermann, S.; PIBOMA content and molar mass on the cure characteristics, rheological and mechanical properties of Weydert, M.; Shaplov, A.S. -

Synergistic Effect of 2-Acrylamido-2-Methyl-1-Propanesulfonic Acid on the Enhanced Conductivity for Fuel Cell at Low Temperature
membranes Article Synergistic Effect of 2-Acrylamido-2-methyl- 1-propanesulfonic Acid on the Enhanced Conductivity for Fuel Cell at Low Temperature Murli Manohar * and Dukjoon Kim * School of Chemical Engineering, Sungkyunkwan University, Suwon, Kyunggi 16419, Korea * Correspondence: [email protected] (M.M.); [email protected] (D.K.) Received: 3 November 2020; Accepted: 10 December 2020; Published: 15 December 2020 Abstract: This present work focused on the aromatic polymer (poly (1,4-phenylene ether-ether-sulfone); SPEES) interconnected/ cross-linked with the aliphatic monomer (2-acrylamido -2-methyl-1-propanesulfonic; AMPS) with the sulfonic group to enhance the conductivity and make it flexible with aliphatic chain of AMPS. Surprisingly, it produced higher conductivity than that of other reported work after the chemical stability was measured. It allows optimizing the synthesis of polymer electrolyte membranes with tailor-made combinations of conductivity and stability. Membrane structure is characterized by 1H NMR and FT-IR. Weight loss of the membrane in Fenton’s reagent is not too high during the oxidative stability test. The thermal stability of the membrane is characterized by TGA and its morphology by SEM and SAXS. The prepared membranes improved 1 proton conductivity up to 0.125 Scm− which is much higher than that of Nafion N115 which is 1 0.059 Scm− . Therefore, the SPEES-AM membranes are adequate for fuel cell at 50 ◦C with reduced relative humidity (RH). Keywords: 2-acrylamido-2-methyl-1-propanesulfonic; proton-exchange membrane; conductivity; cross-linking; temperature 1. Introduction Recently, lots of polymer electrolyte membranes have been prepared from the sulfonation of aromatic polymers such as poly(arylene ether sulfone) [1–4] and modified poly(arylene ether sulfone) [5–7] for the application of electrodialysis and fuel cells as their rigid-rod backbone structures are basically quite stable in thermal and mechanical aspects. -

Quantification of Polycyclic Aromatic Hydrocarbon in Standard
1 Polystyrene plastic: a source and sink for polycyclic aromatic hydrocarbons in the marine 2 environment 3 Chelsea M. Rochman1§, Carlos Manzano2§, Brian T. Hentschel1, Staci L. Massey Simonich2,3, 4 Eunha Hoh4* 5 6 1Department of Biology and Coastal and Marine Institute, San Diego State University, San 7 Diego, CA 8 2Department of Chemistry, Oregon State University, Corvallis, OR 9 3Department of Environmental and Molecular Toxicology, Oregon State University, 10 Corvallis, OR 11 4Graduate School of Public Health, San Diego State University, San Diego, CA 12 13 *Corresponding Author ([email protected]) 14 §Manzano and Rochman are equal contributors to the manuscript. 15 The current address for Chelsea Rochman is the Aquatic Health Program, University of 16 California, Davis, CA and for Carlos Manzano is the Canada Centre for Inland Waters, 17 Burlington, Ontario, Canada 18 Abstract 19 Polycyclic aromatic hydrocarbons (PAHs) on virgin polystyrene (PS) and PS marine 20 debris led us to examine PS as a source and sink for PAHs in the marine environment. At two 21 locations in San Diego Bay, we measured sorption of PAHs to PS pellets, sampling at 0, 1, 3, 6, 22 9 and 12 months. We detected 25 PAHs using a new analytical method with comprehensive two- 23 dimensional gas chromatography coupled to time-of-flight mass spectrometry. Several congeners 1 24 were detected on samples before deployment. After deployment, some concentrations decreased 25 (1,3-dimethylnaphthalene and 2,6-methylnaphthalene) while most increased (2-methylanthracene 26 and all parent PAHs (PPAHs) except fluorene and fluoranthene), suggesting PS debris is a 27 source and sink for PAHs. -

Dynamic Mechanical Analysis of Small Volumes of Styrene Butadiene Rubber at Low Temperatures with Nanoindentation
Application Note Dynamic Mechanical Analysis of Small Volumes of Styrene Butadiene Rubber at Low Temperatures with Nanoindentation Introduction Styrene Butadiene Rubber (SBR) is a synthetic rubber polymer designed to replace natural rubber. SBR is a versatile rubber, and both emulsion- and solution-polymerized SBR are used in tires, binders, batteries, speakers, and construction applications. SBR is commonly used for its elastic properties and abrasion resistance. In this work, the storage modulus of SBR rubber is measured at low temperatures using local dynamic mechanical analysis with a nanoindenter. Figure 1. The chemical formula for Styrene Butadiene Rubber (SBR). Experimental Method A KLA nanoindenter equipped with an InForce 50 actuator and a 50µm diameter flat punch was used to perform local dynamic mechanical analysis (DMA) testing on SBR mounted inside a cold chamber. The cold chamber is capable of cooling a sample down to -60°C while leaving access for nanoindentation. The sample temperature is PID controlled with both heating and cooling elements while the external surfaces of the chamber are maintained at room temperature. Inert gas floods the chamber to mitigate chemical reactions with the atmosphere and to limit the amount of ice formed on the surface of the Figure 2. KLA nanoindentation cold chamber. sample during testing. ProbeDMA is available on all KLA nanoindenters and works Probe DMA Testing Technique with a wide range of flat punch indenter tips. KLA offers a ProbeDMA™ is a nanoindentation technique for measuring sample temperature range from -60°C to 800°C on various frequency-specific viscoelastic material properties. ProbeDMA platforms. This temperature range allows for time-temperature has the advantage of quantifying local mechanical properties superposition experiments and creep compliance function by targeting specific surface locations with the nanoindenter. -

Styrene Styrene Is a Chemical Used to Make Latex, Synthetic Rubber, And
Styrene Styrene is a chemical used to make latex, synthetic rubber, and polystyrene resins. These resins are used to make plastic packaging, disposable cups and containers, insulation, and other products. Styrene is also produced naturally in some plants. How People Are Exposed to Styrene People may be exposed to styrene by breathing it in the air. Styrene is often detected in urban air. It can be found indoors as a result of operating photocopiers and laser printers, and from cigarette smoke. Small amounts may be eaten when styrene migrates into foods from packaging made of polystryrene. How Styrene Affects People's Health The human health effects from exposure to low environmental levels of styrene are unknown. Workers exposed to large amounts of styrene can develop irritation of the eyes and breathing passages. With long-term and large exposures, workers using styrene have had injury to their nervous systems. Levels of Styrene in the U.S. Population In the Fourth National Report on Human Exposure to Environmental Chemicals (Fourth Report), CDC scientists measured styrene in the blood of 1,245 participants aged 20–59 years who took part in the National Health and Nutrition Examination Survey (NHANES) during 2003–2004. The prior survey period of 2001–2002 is also included in the Fourth Report. By measuring styrene in blood, scientists can estimate the amounts of styrene that have entered people’s bodies. CDC researchers found measurable levels of styrene in less than half of the participants. Finding measurable amounts of styrene in the blood does not mean that the levels of styrene cause an adverse health effect. -

An Evaluation of Certain Methods for the Chemical Analysis of Styrene-Butadiene Rubber
NBS TECHNICAL NOTE national tsun Library, E«01 Mnv JUL 3 1969 485 An Evaluation of Certain Methods For the Chemical Analysis of Styrene-Butadiene Rubber T 0F c ^ 0t <5 *. U.S. DEPARTMENT OF COMMERCE if) National Bureau of Standards \ J? * Vau o* NATIONAL BUREAU OF STANDARDS The National Bureau of Standards ' was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation's central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. To this end the Bureau conducts research and provides central national services in four broad program areas. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Radiation Research, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and com- merce. The Institute -

(PAH) from G3MP2B3 Calculations
Paper # P45 Topic: Soot 5th US Combustion Meeting Organized by the Western States Section of the Combustion Institute and Hosted by the University of California at San Diego March 25-28, 2007. Thermochemical Properties of Polycyclic Aromatic Hydrocarbons (PAH) from G3MP2B3 Calculations G. Blanquart and H. Pitsch Department of Mechanical Engineering Stanford University, Stanford, USA In this article, we present a new database of thermodynamic properties for polycyclic aromatic hydro- carbons (PAH). These large aromatic species are formed in very rich premixed flames and in diffusion flames as part of the gas phase chemistry. PAH are commonly assumed to be the intermediates lead- ing to soot formation. Therefore, accurate prediction of their thermodynamic properties is required for modeling soot formation. The present database consists of 46 species ranging from benzene (C6H6) to coronene (C24H12) and includes all the species usually present in chemical mechanisms for soot forma- tion. Geometric molecular structures are optimized at the B3LYP/6-31++G(d,p) level of theory. Heat capacity, entropy and energy content are calculated from these optimized structures. Corrections for hindered rotor are applied based on torsional potentials obtained from second order Møller-Plesset per- turbation (MP2) and Dunning’s consistent basis sets (cc-pVDZ). Enthalpies of formation are calculated using the mixed G3MP2//B3 method. Finally a group correction is applied to account for systematic errors in the G3MP2//B3 computations. The thermodynamic properties for all species are available in NASA polynomial form at the following address: http://www.stanford.edu/groups/pitsch/ 1 Introduction The formation of polycyclic aromatic hydrocarbons (PAH) is a key issue in the understanding of soot formation. -

Exposure to Lead, Mercury, Styrene, and Toluene and Hearing Impairment: Evaluation of Dose- Response Relationships, Regulations, and Controls
Journal of Occupational and Environmental Hygiene ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/uoeh20 Exposure to lead, mercury, styrene, and toluene and hearing impairment: Evaluation of dose- response relationships, regulations, and controls Ehsan Hemmativaghef To cite this article: Ehsan Hemmativaghef (2020): Exposure to lead, mercury, styrene, and toluene and hearing impairment: Evaluation of dose-response relationships, regulations, and controls, Journal of Occupational and Environmental Hygiene, DOI: 10.1080/15459624.2020.1842428 To link to this article: https://doi.org/10.1080/15459624.2020.1842428 Published online: 04 Dec 2020. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=uoeh20 JOURNAL OF OCCUPATIONAL AND ENVIRONMENTAL HYGIENE https://doi.org/10.1080/15459624.2020.1842428 REVIEW Exposure to lead, mercury, styrene, and toluene and hearing impairment: Evaluation of dose-response relationships, regulations, and controls Ehsan Hemmativaghef Faculty of Medicine, School of Population and Public Health, University of British Columbia, Vancouver, BC, Canada ABSTRACT KEYWORDS The risk of hearing loss from exposure to ototoxic chemicals is not reflected in occupational Biological exposure index; exposure limits and most jurisdictions. The aims of this research were to investigate dose- blood lead level; ototoxic response relationships between exposure to lead, mercury, toluene, and styrene and hearing chemicals; pure impairment based on current epidemiological evidence, conduct cross-jurisdictional compar- tone audiometry isons, and investigate control measures for exposure to ototoxic chemicals. Ovid Medline and Ovid Embase databases were used to find relevant publications. -

Sulfonated Styrene-Acrylic Acid Copolymers with Ph-Indicator Property J
http://dx.doi.org/10.21577/0103-5053.20190107 J. Braz. Chem. Soc., Vol. 30, No. 9, 1941-1946, 2019 Article Printed in Brazil - ©2019 Sociedade Brasileira de Química TheSulfonated Sulfonated Styrene-Acrylic Styrene-Acrylic Acid Acid Copolymers Copolymers with with pH-Indicator pH-Indicator Property Property Xin Fan, *,a,b Ping Fan c and Yuehuan Wub aCollege of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, 201620 Shanghai, China bDepartment of Chemistry and Chemical Engineering, Taiyuan Institute of Technology, Shan Xi, 030008 Taiyuan, China cSchool of Materials Science and Engineering, North University of China, Shan Xi, 030051 Taiyuan, China In this study, a polymer pH-indicator was synthesized by sulfonation of poly(styrene-co- acrylic acid) with concentrated sulfuric acid. The synthesized polymer pH-indicator exhibited pH-responsive color changes in aqueous solutions, being purple in alkaline medium (pH < 4), whereas yellow in acidic medium (pH > 9). The sulfonation process extent and polymer color were affected by time and temperature of the process, as well as acrylic acid (AA) content. The addition of acrylic acid was a crucial point for color change due to the facility of cyclic ketone structure to turn into phenol under acidic condition. Compared with low molecule pH-indicators, the sulfonated copolymer was prepared in form of films being repeatedly used as pH-indicator and easily removed from the acid-base medium. Keywords: polymers, sulfonated, pH-indicator, reusability Introduction imidazolium type ionic liquid monomer with acrylonitrile, that was exchanged with sulfonated anionic dyes.23 The pH-indicator has been used in acidic-alkaline solutions color changed from orange red to yellow with the decrease for many years, playing a crucial role in many aspects, of formic acid concentration from 5 to 5 × 10-3 mol L-1.23 such as environmental detection, food freshness and In this study, a simple method was proposed to prepare pH sensors.1-8 It contains low molecular substances, the polymeric pH-indicators. -

Study on Stability and Stability Mechanism of Styrene-Acrylic Emulsion Prepared Using Nanocellulose Modified with Long-Chain Fatty Acids
polymers Article Study on Stability and Stability Mechanism of Styrene-Acrylic Emulsion Prepared Using Nanocellulose Modified with Long-Chain Fatty Acids Heng Zhang 1,2,* , Hongyan Yang 1, Junliang Lu 1, Jinyan Lang 1 and Hongkun Gao 1 1 College of Marine Science and Biological Engineering, Qingdao University of Science & Technology, Qingdao 266042, Shandong, China 2 Key Laboratory of Biomass Chemical Engineering of Ministry of Education, Zhejiang University, Hangzhou 310027, China * Correspondence: [email protected] Received: 18 May 2019; Accepted: 28 June 2019; Published: 3 July 2019 Abstract: In this study, nanocrystalline cellulose (NCC) was grafted with lauric acid, palmitic acid, and stearic acid and used as stabilizer to prepare styrene butyl acrylate emulsion. The properties of the emulsion were determined, and the mechanism of modified NCC (MNCC) stabilized emulsion was analyzed. Results showed that long-chain fatty acids were grafted to NCC through esterification initiated at a low temperature. When the dosage of L-MNCC, P-MNCC, and S-MNCC was 0.05%, the styrene-acrylic emulsion had 92.5%, 94.2%, and 96.3% conversion rates, respectively, and exhibited good dilution, pH, Ca2+, and centrifugal stability. The particle size of styrene-acrylic emulsion was approximately 460 nm, and the absolute value of the Zeta potential increased with the MNCC concentration. According to the images of optical microscopy and the transmission electron microscope, the MNCC was adsorbed onto the surface of styrene-acrylic emulsion droplets. The synergistic effect from the electrostatic repulsion of MNCC, the hydrophile lipophilicity of MNCC, and the spatial hindrance of the MNCC adsorption layer provided good stability for the styrene-acrylic emulsion. -

Synthesis of Poly(2-Acrylamido-2-Methylpropane Sulfonic Acid)
polymers Article Synthesis of Poly(2-Acrylamido-2-Methylpropane Sulfonic Acid) and its Block Copolymers with Methyl Methacrylate and 2-Hydroxyethyl Methacrylate by Quasiliving Radical Polymerization Catalyzed by a Cyclometalated Ruthenium(II) Complex Vanessa Martínez-Cornejo 1 , Joaquin Velázquez-Roblero 1,2, Veronica Rosiles-González 1, Monica Correa-Duran 1, Alejandro Avila-Ortega 2 , Emanuel Hernández-Núñez 3 , Ronan Le Lagadec 4,* and Maria Ortencia González-Díaz 5,* 1 Unidad de Materiales, Centro de Investigación Científica de Yucatán, A.C., Calle 43 No. 130, Chuburná de Hidalgo, C.P. 97205 Mérida, Yucatán, Mexico; [email protected] (V.M.-C.); [email protected] (J.V.-R.); [email protected] (V.R.-G.); [email protected] (M.C.-D.) 2 Facultad de Ingeniería Química, Universidad Autónoma de Yucatán, Periférico Norte Km. 33.5, Chuburná de Hidalgo Inn, C.P. 97203 Mérida, Yucatán, Mexico; [email protected] 3 CONACYT, Departamento de Recursos del Mar, Centro de Investigación y de Estudios Avanzados del IPN, 97310 Unidad Mérida, Yucatán, Mexico; [email protected] 4 Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Ciudad Universitaria, 04510 Ciudad de México, Mexico 5 CONACYT–Centro de Investigación Científica de Yucatán, A.C., Calle 43 No. 130, Chuburná de Hidalgo, 97205 Mérida, Yucatán, Mexico * Correspondence: [email protected] (R.L.L.); [email protected] (M.O.G.-D.) Received: 24 June 2020; Accepted: 20 July 2020; Published: 27 July 2020 Abstract: The first example of quasiliving radical polymerization and copolymerization of 2-acrylamido-2-methylpropane sulfonic acid (AMPS) without previous protection of its strong acid groups catalyzed by [Ru(o-C6H4-2-py)(phen)(MeCN)2]PF6 complex is reported. -
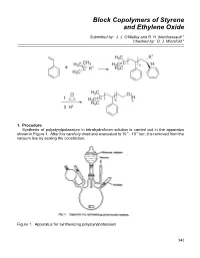
Block Copolymers of Styrene and Ethylene Oxide
Block Copolymers of Styrene and Ethylene Oxide Submitted by: J. J. O'Malley and R. H. Marchessault 1 Checked by: D. J. Worsfold 2 1. Procedure Synthesis of polystyrylpotassium in tetrahydrofuran solution is carried out in the apparatus shown in Figure 1. After it is carefully dried and evacuated to 10-5 - 10-6 torr, it is removed from the vacuum line by sealing the constriction. Figure 1. Apparatus for synthesizing polystyrylpotassium 342 Block Copolymers of Styrene and Ethylene Oxide 343 A red cumylpotassium initiator solution (4.0 ml, 0.095 N, Note 1) in tetrahydrofuran (Note 2) is added to the reaction flask via the breakseal which is broken with a glass-covered iron nail. Any initiator solution remaining in the ampoule can be washed into the flask by cooling the ampoule and condensing solvent in it. After stirring is started, the monomer bulb containing 7.6 g (0.073 mol) of styrene (Note 3) in 38 ml of tetrahydrofuran is sprayed into the initiator solution at 0o through a 0.5 mm bore capillary tube. The nonterminating polymerization is completed almost instantaneously and the red polystyrylpotassium solution is transferred into ampoules, which are sealed from the apparatus at the constrictions and stored in the refrigerator until used (Note 4). The molecular weight of polystyrene prepared in this manner is 20,000.3 The polystyrene-poly(oxyethylene) block copolymer is prepared by adding purified ethylene oxide (Note 5) to a tetrahydrofuran solution of polystyrylpotassium. Caution! Ethylene oxide is very toxic and must be handled with extreme care. Ampoules of polystyrylpotassium (17 ml, 7.6 x 10-3 N in active end-groups) and ethylene oxide (2.5 g, 0.057 mol) are attached to the apparatus shown in Figure 2.