Teacher Background Stem Cells
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Introduction to the Cell Cell History Cell Structures and Functions
Introduction to the cell cell history cell structures and functions CK-12 Foundation December 16, 2009 CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning. Copyright ©2009 CK-12 Foundation This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. Contents 1 Cell structure and function dec 16 5 1.1 Lesson 3.1: Introduction to Cells .................................. 5 3 www.ck12.org www.ck12.org 4 Chapter 1 Cell structure and function dec 16 1.1 Lesson 3.1: Introduction to Cells Lesson Objectives • Identify the scientists that first observed cells. • Outline the importance of microscopes in the discovery of cells. • Summarize what the cell theory proposes. • Identify the limitations on cell size. • Identify the four parts common to all cells. • Compare prokaryotic and eukaryotic cells. Introduction Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. -

Characterization of Embryonic Stem Cell-Differentiated Cells As Mesenchymal Stem Cells
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College Fall 12-2015 Characterization of Embryonic Stem Cell-Differentiated Cells as Mesenchymal Stem Cells Rachael N. Kuehn University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/honors_theses Part of the Cell Biology Commons Recommended Citation Kuehn, Rachael N., "Characterization of Embryonic Stem Cell-Differentiated Cells as Mesenchymal Stem Cells" (2015). Honors Theses. 349. https://aquila.usm.edu/honors_theses/349 This Honors College Thesis is brought to you for free and open access by the Honors College at The Aquila Digital Community. It has been accepted for inclusion in Honors Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi Characterization of Embryonic Stem Cell-Differentiated Cells as Mesenchymal Stem Cells by Rachael Nicole Kuehn A Thesis Submitted to the Honors College of The University of Southern Mississippi in Partial Fulfillment of the Requirements for the Degree of Bachelor of Science in the Department of Biological Sciences December 2015 ii Approved by ______________________________ Yanlin Guo, Ph.D., Thesis Adviser Professor of Biological Sciences ______________________________ Shiao Y. Wang, Ph.D., Chair Department of Biological Sciences ______________________________ Ellen Weinauer, Ph.D., Dean Honors College iii ABSTRACT Embryonic stem cells (ESCs), due to their ability to differentiate into different cell types while still maintaining a high proliferation capacity, have been considered as a potential cell source in regenerative medicine. However, current ESC differentiation methods are low yielding and create heterogeneous cell populations. -

Genomic Profiling Reveals High Frequency of DNA Repair Genetic
www.nature.com/scientificreports OPEN Genomic profling reveals high frequency of DNA repair genetic aberrations in gallbladder cancer Reham Abdel‑Wahab1,9, Timothy A. Yap2, Russell Madison4, Shubham Pant1,2, Matthew Cooke4, Kai Wang4,5,7, Haitao Zhao8, Tanios Bekaii‑Saab6, Elif Karatas1, Lawrence N. Kwong3, Funda Meric‑Bernstam2, Mitesh Borad6 & Milind Javle1,10* DNA repair gene aberrations (GAs) occur in several cancers, may be prognostic and are actionable. We investigated the frequency of DNA repair GAs in gallbladder cancer (GBC), association with tumor mutational burden (TMB), microsatellite instability (MSI), programmed cell death protein 1 (PD‑1), and its ligand (PD‑L1) expression. Comprehensive genomic profling (CGP) of 760 GBC was performed. We investigated GAs in 19 DNA repair genes including direct DNA repair genes (ATM, ATR , BRCA1, BRCA2, FANCA, FANCD2, MLH1, MSH2, MSH6, PALB2, POLD1, POLE, PRKDC, and RAD50) and caretaker genes (BAP1, CDK12, MLL3, TP53, and BLM) and classifed patients into 3 groups based on TMB level: low (< 5.5 mutations/Mb), intermediate (5.5–19.5 mutations/Mb), and high (≥ 19.5 mutations/Mb). We assessed MSI status and PD‑1 & PD‑L1 expression. 658 (86.6%) had at least 1 actionable GA. Direct DNA repair gene GAs were identifed in 109 patients (14.2%), while 476 (62.6%) had GAs in caretaker genes. Both direct and caretaker DNA repair GAs were signifcantly associated with high TMB (P = 0.0005 and 0.0001, respectively). Tumor PD‑L1 expression was positive in 119 (15.6%), with 17 (2.2%) being moderate or high. DNA repair GAs are relatively frequent in GBC and associated with coexisting actionable mutations and a high TMB. -

The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models
biomolecules Review The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models Meera Krishnan 1, Sahil Kumar 1, Luis Johnson Kangale 2,3 , Eric Ghigo 3,4 and Prasad Abnave 1,* 1 Regional Centre for Biotechnology, NCR Biotech Science Cluster, 3rd Milestone, Gurgaon-Faridabad Ex-pressway, Faridabad 121001, India; [email protected] (M.K.); [email protected] (S.K.) 2 IRD, AP-HM, SSA, VITROME, Aix-Marseille University, 13385 Marseille, France; [email protected] 3 Institut Hospitalo Universitaire Méditerranée Infection, 13385 Marseille, France; [email protected] 4 TechnoJouvence, 13385 Marseille, France * Correspondence: [email protected] Abstract: Adult stem cells (ASCs) are the undifferentiated cells that possess self-renewal and differ- entiation abilities. They are present in all major organ systems of the body and are uniquely reserved there during development for tissue maintenance during homeostasis, injury, and infection. They do so by promptly modulating the dynamics of proliferation, differentiation, survival, and migration. Any imbalance in these processes may result in regeneration failure or developing cancer. Hence, the dynamics of these various behaviors of ASCs need to always be precisely controlled. Several genetic and epigenetic factors have been demonstrated to be involved in tightly regulating the proliferation, differentiation, and self-renewal of ASCs. Understanding these mechanisms is of great importance, given the role of stem cells in regenerative medicine. Investigations on various animal models have played a significant part in enriching our knowledge and giving In Vivo in-sight into such ASCs regulatory mechanisms. In this review, we have discussed the recent In Vivo studies demonstrating the role of various genetic factors in regulating dynamics of different ASCs viz. -

Mitosis Vs. Meiosis
Mitosis vs. Meiosis In order for organisms to continue growing and/or replace cells that are dead or beyond repair, cells must replicate, or make identical copies of themselves. In order to do this and maintain the proper number of chromosomes, the cells of eukaryotes must undergo mitosis to divide up their DNA. The dividing of the DNA ensures that both the “old” cell (parent cell) and the “new” cells (daughter cells) have the same genetic makeup and both will be diploid, or containing the same number of chromosomes as the parent cell. For reproduction of an organism to occur, the original parent cell will undergo Meiosis to create 4 new daughter cells with a slightly different genetic makeup in order to ensure genetic diversity when fertilization occurs. The four daughter cells will be haploid, or containing half the number of chromosomes as the parent cell. The difference between the two processes is that mitosis occurs in non-reproductive cells, or somatic cells, and meiosis occurs in the cells that participate in sexual reproduction, or germ cells. The Somatic Cell Cycle (Mitosis) The somatic cell cycle consists of 3 phases: interphase, m phase, and cytokinesis. 1. Interphase: Interphase is considered the non-dividing phase of the cell cycle. It is not a part of the actual process of mitosis, but it readies the cell for mitosis. It is made up of 3 sub-phases: • G1 Phase: In G1, the cell is growing. In most organisms, the majority of the cell’s life span is spent in G1. • S Phase: In each human somatic cell, there are 23 pairs of chromosomes; one chromosome comes from the mother and one comes from the father. -

Boosting the Cellular Potency of Embryonic Stem Cells by Spliceosome Targeting ✉ Wilfried A
Signal Transduction and Targeted Therapy www.nature.com/sigtrans RESEARCH HIGHLIGHT OPEN Boosting the cellular potency of embryonic stem cells by spliceosome targeting ✉ Wilfried A. Kues1 Signal Transduction and Targeted Therapy (2021) 6:324; https://doi.org/10.1038/s41392-021-00743-9 In recent work published in Cell, Shen et al.1 identified transfected ES cells with short interfering RNAs against different spliceosome inhibition in embryonic stem (ES) cells as a key spliceosome transcripts (the spliceosome consisted of 5 core and mechanism for the transition from pluri- to totipotency. Spliceo- several cofactor subunits, here 14 transcripts were targeted), some inhibition, achieved by RNA interference or the chemical respectively. Transient repression of 10 of the 14 splicing factors inhibitor pladienolide B, may gain widespread relevance to the resulted in ES cells, which maintained the typical colony culture of totipotent ES cells, in vitro differentiation of extra- morphology, however, pluripotent marker genes—Oct4 (Pou5f1), embryonal tissue and organoids, translation to the maintenance of Nanog, Sox2, Zfp42 and others—became down-regulated, at the pluripotent cells of other mammal species, including humans, and same time marker genes of totipotency—particularly Zscan4s and a better molecular understanding of cellular potency in stem cells MERVL—were up-regulated. Zscan4s (Zink finger and SCAN and cancer. domain containing 4) is a transcription factor and MERVL (murine The first successful isolation and maintenance of ES derived endogenous retrovirus L) an endogenous retrovirus with a usually fi 1234567890();,: from the inner cell mass (ICM) of murine blastocyst stages was restricted expression to 2-cell embryos. These results were veri ed described in 1981,2 and since then acted as game changer for by supplementing the culture medium with pladienolide B, a genetic studies in this mammalian model organism. -

Epigenetic Metabolites License Stem Cell States
CHAPTER SIX Epigenetic metabolites license stem cell states Logeshwaran Somasundarama,b,†, Shiri Levya,b,†, Abdiasis M. Husseina,b, Devon D. Ehnesa,b, Julie Mathieub,c, Hannele Ruohola-Bakera,b,∗ aDepartment of Biochemistry, University of Washington, Seattle, WA, United States bInstitute for Stem Cell and Regenerative Medicine, University of Washington, Seattle, WA, United States cDepartment of Comparative Medicine, University of Washington, Seattle, WA, United States ∗ Corresponding author: e-mail address: [email protected] Contents 1. Introduction 210 2. Stem cell energetics 210 3. Metabolism of quiescent stem cells 212 3.1 Adult stem cells 212 3.2 Pluripotent stem cell quiescence, diapause 216 4. Metabolism of active stem cells 217 4.1 Metabolism after fertilization 217 4.2 Metabolism of pre-implantation and post-implantation pluripotent stem cells 218 4.3 Metabolism of actively cycling adult stem cells: MSC as case-study 220 5. HIF, the master regulator of metabolism 222 6. Epigenetic signatures and epigenetic metabolites 224 6.1 Epigenetic signatures of naïve and primed pluripotent stem cells 224 6.2 Epigenetic signatures of adult stem cells 227 6.3 Epigenetic metabolites 228 7. Conclusion 229 Acknowledgments 230 References 230 Further reading 240 Abstract It has become clear during recent years that stem cells undergo metabolic remodeling during their activation process. While these metabolic switches take place in pluripotency as well as adult stem cell populations, the rules that govern the switch are not clear. † Equal contribution. # Current Topics in Developmental Biology, Volume 138 2020 Elsevier Inc. 209 ISSN 0070-2153 All rights reserved. https://doi.org/10.1016/bs.ctdb.2020.02.003 210 Logeshwaran Somasundaram et al. -
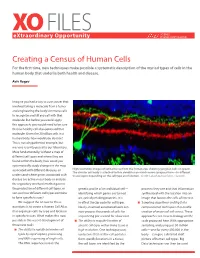
Creating a Census of Human Cells
XO FILES SCIENCE eXtraordinary Opportunity PHILANTHROPY ALLIANCE Creating a Census of Human Cells For the first time, new techniques make possible a systematic description of the myriad types of cells in the human body that underlie both health and disease. Aviv Regev Imagine you had a way to cure cancer that involved taking a molecule from a tumor and engineering the body’s immune cells to recognize and kill any cell with that molecule. But before you could apply this approach, you would need to be sure that no healthy cell also expressed that molecule. Given the 20 trillion cells in a human body, how would you do that? This is not a hypothetical example, but was one recently posed to our laboratory. More fundamentally, without a map of different cell types and where they are found within the body, how could you systematically study changes in the map associated with different diseases, or High resolution images of retinal tissue from the human eye, showing ganglion cells (in green). The circular cell body is attached to thin dendrites on which nerve synapses form—in different understand where genes associated with tissue layers depending on the cell type and function. Credit: Lab of Joshua Sanes, Harvard. disease are active in our body or analyze the regulatory mechanisms that govern the production of different cell types, or genetic profile of an individual cell— proteins they use and that information sort out how different cell types combine identifying which genes are turned synthesized with the location into an to form specific tissues? on, actively making proteins. -

Exploring the Interplay of Telomerase Reverse Transcriptase and Β-Catenin in Hepatocellular Carcinoma
cancers Review Exploring the Interplay of Telomerase Reverse Transcriptase and β-Catenin in Hepatocellular Carcinoma Srishti Kotiyal and Kimberley Jane Evason * Department of Oncological Sciences, Department of Pathology, and Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-801-587-4606 Simple Summary: Liver cancer is one of the deadliest human cancers. Two of the most common molecular aberrations in liver cancer are: (1) activating mutations in the gene encoding β-catenin (CTNNB1); and (2) promoter mutations in telomerase reverse transcriptase (TERT). Here, we review recent findings regarding the interplay between TERT and β-catenin in order to better understand their role in liver cancer. Abstract: Hepatocellular carcinoma (HCC) is one of the deadliest human cancers. Activating muta- tions in the telomerase reverse transcriptase (TERT) promoter (TERTp) and CTNNB1 gene encoding β-catenin are widespread in HCC (~50% and ~30%, respectively). TERTp mutations are predicted to increase TERT transcription and telomerase activity. This review focuses on exploring the role of TERT and β-catenin in HCC and the current findings regarding their interplay. TERT can have contradictory effects on tumorigenesis via both its canonical and non-canonical functions. As a critical regulator of proliferation and differentiation in progenitor and stem cells, activated β-catenin drives HCC; however, inhibiting endogenous β-catenin can also have pro-tumor effects. Clinical studies revealed a significant concordance between TERTp and CTNNB1 mutations in HCC. In Citation: Kotiyal, S.; Evason, K.J. stem cells, TERT acts as a co-factor in β-catenin transcriptional complexes driving the expression Exploring the Interplay of Telomerase of WNT/β-catenin target genes, and β-catenin can bind to the TERTp to drive its transcription. -

Telomere and Telomerase in Oncology
Cell Research (2002); 12(1):1-7 http://www.cell-research.com REVIEW Telomere and telomerase in oncology JIAO MU*, LI XIN WEI International Joint Cancer Institute, Second Military Medical University, Shanghai 200433, China ABSTRACT Shortening of the telomeric DNA at the chromosome ends is presumed to limit the lifespan of human cells and elicit a signal for the onset of cellular senescence. To continually proliferate across the senescent checkpoint, cells must restore and preserve telomere length. This can be achieved by telomerase, which has the reverse transcriptase activity. Telomerase activity is negative in human normal somatic cells but can be detected in most tumor cells. The enzyme is proposed to be an essential factor in cell immortalization and cancer progression. In this review we discuss the structure and function of telomere and telomerase and their roles in cell immortalization and oncogenesis. Simultaneously the experimental studies of telomerase assays for cancer detection and diagnosis are reviewed. Finally, we discuss the potential use of inhibitors of telomerase in anti-cancer therapy. Key words: Telomere, telomerase, cancer, telomerase assay, inhibitor. Telomere and cell replicative senescence base pairs of the end of telomeric DNA with each Telomeres, which are located at the end of round of DNA replication. Hence, the continual chromosome, are crucial to protect chromosome cycles of cell growth and division bring on progress- against degeneration, rearrangment and end to end ing telomere shortening[4]. Now it is clear that te- fusion[1]. Human telomeres are tandemly repeated lomere shortening is responsible for inducing the units of the hexanucleotide TTAGGG. The estimated senescent phenotype that results from repeated cell length of telomeric DNA varies from 2 to 20 kilo division, but the mechanism how a short telomere base pairs, depending on factors such as tissue type induces the senescence is still unknown. -

Robustness and Timing of Cellular Differentiation Through Population-Based Symmetry Breaking
AR TI CLE Robustness AND TIMING OF CELLULAR DIFFERENTIATION THROUGH population-based SYMMETRY BREAKING Angel StanoeV1 | Christian Schröter1 | Aneta KOSESKA1∗ 1Department OF Systemic Cell Biology, Max Planck Institute OF Molecular PhYSIOLOGY, Although COORDINATED BEHAVIOR OF MULTICELLULAR ORGANISMS Dortmund, GermanY RELIES ON INTERCELLULAR communication, THE DYNAMICAL mech- Correspondence ANISM THAT UNDERLIES SYMMETRY BREAKING IN HOMOGENEOUS Email: populations, THEREBY GIVING RISE TO HETEROGENEOUS CELL TYPES ∗[email protected] REMAINS UNCLEAR. In CONTRAST TO THE PREVALENT cell-intrinsic VIEW OF DIFFERENTIATION WHERE MULTISTABILITY ON THE SINGLE CELL LEVEL IS A NECESSARY pre-requisite, WE PROPOSE THAT het- EROGENEOUS CELLULAR IDENTITIES EMERGE AND ARE MAINTAINED COOPERATIVELY ON A POPULATION LEVel. Identifying A GENERIC SYMMETRY BREAKING mechanism, WE DEMONSTRATE THAT ROBUST PROPORTIONS BETWEEN DIFFERENTIATED CELL TYPES ARE INTRINSIC FEATURES OF THIS INHOMOGENEOUS STEADY STATE solution. Crit- ICAL ORGANIZATION IN THE VICINITY OF THE SYMMETRY BREAKING BIFURCATION ON THE OTHER hand, SUGGESTS THAT TIMING OF cel- LULAR DIFFERENTIATION EMERGES FOR GROWING POPULATIONS IN A self-organized MANNER. Setting A CLEAR DISTINCTION BETWEEN pre-eXISTING ASYMMETRIES AND SYMMETRY BREAKING EVents, WE THUS DEMONSTRATE THAT EMERGENT SYMMETRY breaking, IN CONJUNCTION WITH ORGANIZATION NEAR CRITICALITY NECESSARILY LEADS TO ROBUSTNESS AND ACCURACY DURING DEVelopment. KEYWORDS SYMMETRY breaking; differentiation; INHOMOGENEOUS STEADY STATE Abbreviations: -

Translational Applications of Adult Stem Cell-Derived Organoids Jarno Drost1,2,* and Hans Clevers1,2,3,‡
© 2017. Published by The Company of Biologists Ltd | Development (2017) 144, 968-975 doi:10.1242/dev.140566 PRIMER Translational applications of adult stem cell-derived organoids Jarno Drost1,2,* and Hans Clevers1,2,3,‡ ABSTRACT of organotypic organoids from single Lgr5-positive ISCs (Sato Adult stem cells from a variety of organs can be expanded long- et al., 2009). These organoids contained all cell types of the ‘ ’ term in vitro as three-dimensional organotypic structures termed intestinal epithelium and were structured into proliferative crypt ‘ ’ organoids. These adult stem cell-derived organoids retain their organ and differentiated villus compartments, thereby retaining the identity and remain genetically stable over long periods of time. The architecture of the native intestine (Sato et al., 2009). In addition ability to grow organoids from patient-derived healthy and diseased to Wnt/R-spondin, epidermal growth factor (EGF) (Dignass and tissue allows for the study of organ development, tissue homeostasis Sturm, 2001), noggin (BMP inhibitor) (Haramis et al., 2004) and an and disease. In this Review, we discuss the generation of adult artificial laminin-rich extracellular matrix (provided by Matrigel) stem cell-derived organoid cultures and their applications in in vitro complemented the cocktail for successful in vitro propagation of disease modeling, personalized cancer therapy and regenerative mouse ISC-derived organoids (Sato et al., 2009). At the same time, medicine. Ootani et al. reported another Wnt-driven in vitro culture of ISCs (Ootani et al., 2009). In contrast to Sato et al., this culture was not KEY WORDS: Adult stem cells, Organoids, Disease modelling, based on defined growth factors.