ING5 Activity in Self-Renewal of Glioblastoma Stem Cells Via Calcium and Follicle Stimulating Hormone Pathways
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Expression Pattern and Level of ING5 Protein in Normal and Cancer Tissues
ONCOLOGY LETTERS 17: 63-68, 2019 Expression pattern and level of ING5 protein in normal and cancer tissues XUE-FENG YANG1, DAO-FU SHEN1, SHUANG ZHAO1, TIAN-REN REN2, YANG GAO1, SHUAI SHI1, JI-CHENG WU1, HONG-ZHI SUN1 and HUA-CHUAN ZHENG1,3 1Cancer Center and Key Laboratory of Brain and Spinal Cord Injury of Liaoning Province, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning 121001;2 Jilin Province Forestry Bureau, Linjiang, Jilin 134600; 3Institute of Life Sciences, Jinzhou Medical University, Jinzhou, Liaoning 121001, P.R. China Received February 21, 2016; Accepted February 13, 2017 DOI: 10.3892/ol.2018.9581 Abstract. Inhibitor of growth family 5 (ING5) functions as may be involved in cell regeneration and be associated with a type-II tumor suppressor gene and exerts an important role colorectal carcinogenesis. in DNA repair, apoptotic induction, proliferative inhibition, chromatin remodeling and the invasion process. In the present Introduction study, immunohistochemistry was performed to characterize the expression profile of ING5 protein on a tissue microarray Inhibitor of growth family 5 (ING5) is a member of the ING containing mouse and human normal tissues, and human protein family and functions as a type-II tumor suppressor cancer tissues, including hepatocellular (n=62), renal clear cell gene. Human ING5 is mapped to chromosome 2q37.3 and (n=62), pancreatic (n=62), esophageal squamous cell (n=45), encodes a 28-kDa protein with 240 amino acids (1). As indi- cervical squamous cell (n=31), breast (n=144), gastric (n=196), cated in Fig. 1, ING5 protein consists of a number of domains, colorectal (n=96), endometrial (n=96) and lung carcinoma including leucine zipper like (LZL), novel conserved region (n=192). -

Functional Roles of Bromodomain Proteins in Cancer
cancers Review Functional Roles of Bromodomain Proteins in Cancer Samuel P. Boyson 1,2, Cong Gao 3, Kathleen Quinn 2,3, Joseph Boyd 3, Hana Paculova 3 , Seth Frietze 3,4,* and Karen C. Glass 1,2,4,* 1 Department of Pharmaceutical Sciences, Albany College of Pharmacy and Health Sciences, Colchester, VT 05446, USA; [email protected] 2 Department of Pharmacology, Larner College of Medicine, University of Vermont, Burlington, VT 05405, USA; [email protected] 3 Department of Biomedical and Health Sciences, University of Vermont, Burlington, VT 05405, USA; [email protected] (C.G.); [email protected] (J.B.); [email protected] (H.P.) 4 University of Vermont Cancer Center, Burlington, VT 05405, USA * Correspondence: [email protected] (S.F.); [email protected] (K.C.G.) Simple Summary: This review provides an in depth analysis of the role of bromodomain-containing proteins in cancer development. As readers of acetylated lysine on nucleosomal histones, bromod- omain proteins are poised to activate gene expression, and often promote cancer progression. We examined changes in gene expression patterns that are observed in bromodomain-containing proteins and associated with specific cancer types. We also mapped the protein–protein interaction network for the human bromodomain-containing proteins, discuss the cellular roles of these epigenetic regu- lators as part of nine different functional groups, and identify bromodomain-specific mechanisms in cancer development. Lastly, we summarize emerging strategies to target bromodomain proteins in cancer therapy, including those that may be essential for overcoming resistance. Overall, this review provides a timely discussion of the different mechanisms of bromodomain-containing pro- Citation: Boyson, S.P.; Gao, C.; teins in cancer, and an updated assessment of their utility as a therapeutic target for a variety of Quinn, K.; Boyd, J.; Paculova, H.; cancer subtypes. -

The Tumor Suppressor ING5 Is a Dimeric, Bivalent Recognition Molecule of the Histone H3k4me3 Mark
Article The Tumor Suppressor ING5 Is a Dimeric, Bivalent Recognition Molecule of the Histone H3K4me3 Mark Georgina Ormaza 1,†, Jhon A. Rodríguez 1,†, Alain Ibáñez de Opakua 1, Nekane Merino 1, Maider Villate 1, Irantzu Gorroño 1, Miriam Rábano 1, Ignacio Palmero 2, Marta Vilaseca 3, Robert Kypta 1,4, María d.M. Vivanco 1, Adriana L. Rojas 1 and Francisco J. Blanco 1,5 1 - CIC bioGUNE, Parque Tecnológico de Bizkaia, 48160 Derio, Spain 2 - Instituto de Investigaciones Biomédicas “Alberto Sols”, CSIC-UAM, 28029 Madrid, Spain 3 - Institute for Research in Biomedicine, 08028 Barcelona, Spain 4 - Department of Surgery and Cancer, Imperial College London, London, W12 0NN, UK 5 - IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain Correspondence to Francisco J. Blanco: CIC bioGUNE, Parque Tecnológico de Bizkaia, 48160 Derio, Spain. [email protected] https://doi.org/10.1016/j.jmb.2019.04.018 Edited by M. Guss Abstract The INhibitor of Growth (ING) family of tumor suppressors regulates the transcriptional state of chromatin by recruiting remodeling complexes to sites with histone H3 trimethylated at lysine 4 (H3K4me3). This modification is recognized by the plant homeodomain (PHD) present at the C-terminus of the five ING proteins. ING5 facilitates histone H3 acetylation by the HBO1 complex, and also H4 acetylation by the MOZ/ MORF complex. We show that ING5 forms homodimers through its N-terminal domain, which folds independently into an elongated coiled-coil structure. The central region of ING5, which contains the nuclear localization sequence, is flexible and disordered, but it binds dsDNA with micromolar affinity. NMR analysis of the full-length protein reveals that the two PHD fingers of the dimer are chemically equivalent and independent of the rest of the molecule, and they bind H3K4me3 in the same way as the isolated PHD. -

Modes of Interaction of KMT2 Histone H3 Lysine 4 Methyltransferase/COMPASS Complexes with Chromatin
cells Review Modes of Interaction of KMT2 Histone H3 Lysine 4 Methyltransferase/COMPASS Complexes with Chromatin Agnieszka Bochy ´nska,Juliane Lüscher-Firzlaff and Bernhard Lüscher * ID Institute of Biochemistry and Molecular Biology, Medical School, RWTH Aachen University, Pauwelsstrasse 30, 52057 Aachen, Germany; [email protected] (A.B.); jluescher-fi[email protected] (J.L.-F.) * Correspondence: [email protected]; Tel.: +49-241-8088850; Fax: +49-241-8082427 Received: 18 January 2018; Accepted: 27 February 2018; Published: 2 March 2018 Abstract: Regulation of gene expression is achieved by sequence-specific transcriptional regulators, which convey the information that is contained in the sequence of DNA into RNA polymerase activity. This is achieved by the recruitment of transcriptional co-factors. One of the consequences of co-factor recruitment is the control of specific properties of nucleosomes, the basic units of chromatin, and their protein components, the core histones. The main principles are to regulate the position and the characteristics of nucleosomes. The latter includes modulating the composition of core histones and their variants that are integrated into nucleosomes, and the post-translational modification of these histones referred to as histone marks. One of these marks is the methylation of lysine 4 of the core histone H3 (H3K4). While mono-methylation of H3K4 (H3K4me1) is located preferentially at active enhancers, tri-methylation (H3K4me3) is a mark found at open and potentially active promoters. Thus, H3K4 methylation is typically associated with gene transcription. The class 2 lysine methyltransferases (KMTs) are the main enzymes that methylate H3K4. KMT2 enzymes function in complexes that contain a necessary core complex composed of WDR5, RBBP5, ASH2L, and DPY30, the so-called WRAD complex. -

Cover Petra B
UvA-DARE (Digital Academic Repository) The role of Polycomb group proteins throughout development : f(l)avoring repression van der Stoop, P.M. Link to publication Citation for published version (APA): van der Stoop, P. M. (2009). The role of Polycomb group proteins throughout development : f(l)avoring repression. Amsterdam: Nederlands Kanker Instituut - Antoni Van Leeuwenhoekziekenhuis. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (http://dare.uva.nl) Download date: 27 Oct 2019 Chapter 4 Ubiquitin E3 Ligase Ring1b/Rnf2 of Polycomb Repressive Complex 1 Contributes to Stable Maintenance of Mouse Embryonic Stem Cells Petra van der Stoop*, Erwin A. Boutsma*, Danielle Hulsman, Sonja Noback, Mike Heimerikx, Ron M. Kerkhoven, J. Willem Voncken, Lodewyk F.A. Wessels, Maarten van Lohuizen * These authors contributed equally to this work Adapted from: PLoS ONE (2008) 3(5): e2235 Ring1b regulates ES cell fate Ubiquitin E3 Ligase Ring1b/Rnf2 of Polycomb Repressive Complex 1 Contributes to Stable Maintenance of Mouse Embryonic Stem Cells Petra van der Stoop1*, Erwin A. -

Recognition of Cancer Mutations in Histone H3K36 by Epigenetic Writers and Readers Brianna J
EPIGENETICS https://doi.org/10.1080/15592294.2018.1503491 REVIEW Recognition of cancer mutations in histone H3K36 by epigenetic writers and readers Brianna J. Kleina, Krzysztof Krajewski b, Susana Restrepoa, Peter W. Lewis c, Brian D. Strahlb, and Tatiana G. Kutateladzea aDepartment of Pharmacology, University of Colorado School of Medicine, Aurora, CO, USA; bDepartment of Biochemistry & Biophysics, The University of North Carolina School of Medicine, Chapel Hill, NC, USA; cWisconsin Institute for Discovery, University of Wisconsin, Madison, WI, USA ABSTRACT ARTICLE HISTORY Histone posttranslational modifications control the organization and function of chromatin. In Received 30 May 2018 particular, methylation of lysine 36 in histone H3 (H3K36me) has been shown to mediate gene Revised 1 July 2018 transcription, DNA repair, cell cycle regulation, and pre-mRNA splicing. Notably, mutations at or Accepted 12 July 2018 near this residue have been causally linked to the development of several human cancers. These KEYWORDS observations have helped to illuminate the role of histones themselves in disease and to clarify Histone; H3K36M; cancer; the mechanisms by which they acquire oncogenic properties. This perspective focuses on recent PTM; methylation advances in discovery and characterization of histone H3 mutations that impact H3K36 methyla- tion. We also highlight findings that the common cancer-related substitution of H3K36 to methionine (H3K36M) disturbs functions of not only H3K36me-writing enzymes but also H3K36me-specific readers. The latter case suggests that the oncogenic effects could also be linked to the inability of readers to engage H3K36M. Introduction from yeast to humans and has been shown to have a variety of functions that range from the control Histone proteins are main components of the of gene transcription and DNA repair, to cell cycle nucleosome, the fundamental building block of regulation and nutrient stress response [8]. -

The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies The Vault: Electronic Theses and Dissertations 2016 The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells Wang, Fangwu Jr Wang, F. J. (2016). The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells (Unpublished master's thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/28327 http://hdl.handle.net/11023/3233 master thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca UNIVERSITY OF CALGARY The Role of ING5 in Maintaining Stemness of Brain Tumor Initiating Cells by Fangwu Wang A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE GRADUATE PROGRAM IN BIOCHEMISTRY AND MOLECULAR BIOLOGY CALGARY, ALBERTA AUGUST, 2016 © Fangwu Wang 2016 Abstract Brain tumor initiating cells (BTICs) are believed to account for the recurrence of glioblastomas following treatment. Recent studies have shown that the stemness of BTICs and intratumoral differentiation hierarchy are determined largely on the epigenetic level. The ING family of epigenetic regulators function in diverse growth regulatory, metastasis and chemoresistance pathways, through targeting different histone acetyltransferase (HAT) and histone deacetylase (HDAC) complexes to the H3K4me3 mark to alter histone acetylation. -

Further Delineation of the Clinical Spectrum of KAT6B Disorders and Allelic Series of Pathogenic Variants
ARTICLE © American College of Medical Genetics and Genomics Further delineation of the clinical spectrum of KAT6B disorders and allelic series of pathogenic variants Li Xin Zhang1, Gabrielle Lemire, MD2, Claudia Gonzaga-Jauregui, PhD3, Sirinart Molidperee, Gr. Cert1, Carolina Galaz-Montoya, MD3, David S. Liu, MD3, Alain Verloes, MD PhD4, Amelle G. Shillington, DO5, Kosuke Izumi, MD PhD6, Alyssa L. Ritter, MS LCGC7, Beth Keena, MS LCGC6, Elaine Zackai, MD7,8, Dong Li, PhD9, Elizabeth Bhoj, MD PhD7, Jennifer M. Tarpinian, MS CGC10, Emma Bedoukian, MS LCGC10, Mary K. Kukolich, MD11, A. Micheil Innes, MD12, Grace U. Ediae, BSc13, Sarah L. Sawyer, MD PhD14, Karippoth Mohandas Nair, MD15, Para Chottil Soumya, MD15, Kinattinkara R. Subbaraman, MD15, Frank J. Probst, MD PhD3,16, Jennifer A. Bassetti, MD3,16, Reid V. Sutton, MD3,16, Richard A. Gibbs, PhD3, Chester Brown, MD PhD17, Philip M. Boone, MD PhD18, Ingrid A. Holm, MD MPH18, Marco Tartaglia, PhD19, Giovanni Battista Ferrero, MD PhD20, Marcello Niceta, MD PhD19, Maria Lisa Dentici, MD19, Francesca Clementina Radio, MD PhD19, Boris Keren, MD21, Constance F. Wells, MD22, Christine Coubes, MD22, Annie Laquerrière, MD PhD23, Jacqueline Aziza, MD24, Charlotte Dubucs, MD24, Sheela Nampoothiri, MD25, David Mowat, MD26, Millan S. Patel, MD27, Ana Bracho, MD28, Francisco Cammarata-Scalisi, MD29, Alper Gezdirici, MD30, Alberto Fernandez-Jaen, MD31, Natalie Hauser, MD32, Yuri A. Zarate, MD33, Katherine A. Bosanko, MS CGC33, Klaus Dieterich, MD PhD34, John C. Carey, MD MPH35, Jessica X. Chong, PhD36,37, Deborah A. Nickerson, PhD37,38, Michael J. Bamshad, MD36,37, Brendan H. Lee, MD PhD3, Xiang-Jiao Yang, PhD39, James R. -

Open Data for Differential Network Analysis in Glioma
International Journal of Molecular Sciences Article Open Data for Differential Network Analysis in Glioma , Claire Jean-Quartier * y , Fleur Jeanquartier y and Andreas Holzinger Holzinger Group HCI-KDD, Institute for Medical Informatics, Statistics and Documentation, Medical University Graz, Auenbruggerplatz 2/V, 8036 Graz, Austria; [email protected] (F.J.); [email protected] (A.H.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 27 October 2019; Accepted: 3 January 2020; Published: 15 January 2020 Abstract: The complexity of cancer diseases demands bioinformatic techniques and translational research based on big data and personalized medicine. Open data enables researchers to accelerate cancer studies, save resources and foster collaboration. Several tools and programming approaches are available for analyzing data, including annotation, clustering, comparison and extrapolation, merging, enrichment, functional association and statistics. We exploit openly available data via cancer gene expression analysis, we apply refinement as well as enrichment analysis via gene ontology and conclude with graph-based visualization of involved protein interaction networks as a basis for signaling. The different databases allowed for the construction of huge networks or specified ones consisting of high-confidence interactions only. Several genes associated to glioma were isolated via a network analysis from top hub nodes as well as from an outlier analysis. The latter approach highlights a mitogen-activated protein kinase next to a member of histondeacetylases and a protein phosphatase as genes uncommonly associated with glioma. Cluster analysis from top hub nodes lists several identified glioma-associated gene products to function within protein complexes, including epidermal growth factors as well as cell cycle proteins or RAS proto-oncogenes. -

Recombinant BRPF1 (627-746) Protein Catalog No: 31375, 31875 Quantity: 100, 1000 Μg Lot No: 32717002 Concentration: 1.3 Μg/Μl Expressed In: E
Recombinant BRPF1 (627-746) protein Catalog No: 31375, 31875 Quantity: 100, 1000 µg Lot No: 32717002 Concentration: 1.3 µg/µl Expressed In: E. coli Source: Human Buffer Contents: Recombinant BRPF1 (627-746) protein expressed in E. coli and provided in 25 mM Tris pH 8, 300 mM NaCl and 10% glycerol. Background: Bromodomain and PHD finger containing (BRPF1) protein is a component of the MOZ/MORF complex which has histone H3 acetyltransferase activity. The bromodomain of BRPF1 recognizes acetylated histone lysine residues and function as a ‘reader’ of these epigenetic histone marks to regulate chromatin structure and gene expression by linking associated proteins to the recognized acetylated nucleosomal targets. BRPF1 is known to positively regulate the transcription of RUNX1 and RUNX2. Protein Details: The peptide corresponding to amino acids 627 - 746 that contains the bromodomain sequences of BRPF1 (accession number NM_001003694.1) was expressed in E. coli and contains an N-terminal His-Tag and C- terminal FLAG-Tag with an observed molecular weight of 20.3 kDa. The recombinant protein is >90% pure by SDS- PAGE. Application Notes: Recombinant BRPF1 (627-746) is suitable for use in binding assays, inhibitor screening, and selectivity profiling. Storage and Guarantee: Recombinant proteins in solution are temperature sensitive and must be stored at -80°C to prevent degradation. Avoid repeated freeze/thaw cycles and keep on ice when not in storage. This product is guaranteed for 6 months from date of receipt. This product is for research use only and is not for use in diagnostic procedures. North America 877 222 9543 • Europe +32 (0)2 653 0001 • Japan +81 (0)3 5225 3638 • www.activemotif.com Recombinant BRPF1 (627-746) protein gel. -
Download Validation Data
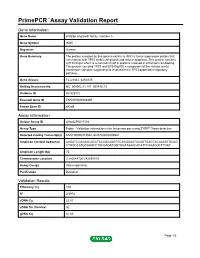
PrimePCR™Assay Validation Report Gene Information Gene Name inhibitor of growth family, member 5 Gene Symbol ING5 Organism Human Gene Summary The protein encoded by this gene is similar to ING1 a tumor suppressor protein that can interact with TP53 inhibit cell growth and induce apoptosis. This protein contains a PHD-finger which is a common motif in proteins involved in chromatin remodeling. This protein can bind TP53 and EP300/p300 a component of the histone acetyl transferase complex suggesting its involvement in TP53-dependent regulatory pathway. Gene Aliases FLJ23842, p28ING5 RefSeq Accession No. NC_000002.11, NT_005416.13 UniGene ID Hs.529172 Ensembl Gene ID ENSG00000168395 Entrez Gene ID 84289 Assay Information Unique Assay ID qHsaCIP0031594 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000313552, ENST00000406941 Amplicon Context Sequence AAGATCCAGAACGCCTACAGCAAGTGCAAGGAATACAGTGACGACAAAGTGCAG CTGGCCATGCAGACCTACGAGATGGTGGATAAACACATTCGAAGGCTTGAT Amplicon Length (bp) 75 Chromosome Location 2:242648720-242650818 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 100 R2 0.9978 cDNA Cq 22.07 cDNA Tm (Celsius) 82 gDNA Cq 41.86 Page 1/5 PrimePCR™Assay Validation Report Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report ING5, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak -

Deficiency of Intellectual Disability-Related Gene Brpf1 Reduced Inhibitory
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.11.426233; this version posted January 12, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Deficiency of intellectual disability-related gene Brpf1 reduced inhibitory 2 neurotransmission and Map2k7 expression in GABAergic interneurons 3 Jingli Cao1, Weiwei Xian1, Maierdan Palihati1, Yu Zhu1, Guoxiang Wang2, Yunli Xie2, 4 Guomin Zhou1,3#, Linya You1# 5 6 1Department of Human Anatomy & Histoembryology, School of Basic Medical 7 Sciences, Fudan University, Shanghai 200032, China 8 2Institutes of Brain Sciences, Fudan University, Shanghai 200032, China 9 3Key Laboratory of Medical Imaging Computing and Computer Assisted Intervention 10 of Shanghai, Shanghai 200032, China 11 12 #Correspondences to: [email protected]; [email protected]. 13 14 Conflict of interest: The authors declare no conflicts of interest. 15 16 Running Title: Brpf1 loss reduced neurotransmission 17 18 Keywords: intellectual disability; Brpf1; MGE-derived GABAergic interneurons; 19 differentiation; dendritic morphology, electrophysiology; in vivo migration; 20 mRNA-Seq 21 22 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.11.426233; this version posted January 12, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 23 Abstract 24 Intellectual disability is closely related to impaired GABA neurotransmission.