Multiple Choice Questions on Monoclonal Antibodies 1
Total Page:16
File Type:pdf, Size:1020Kb
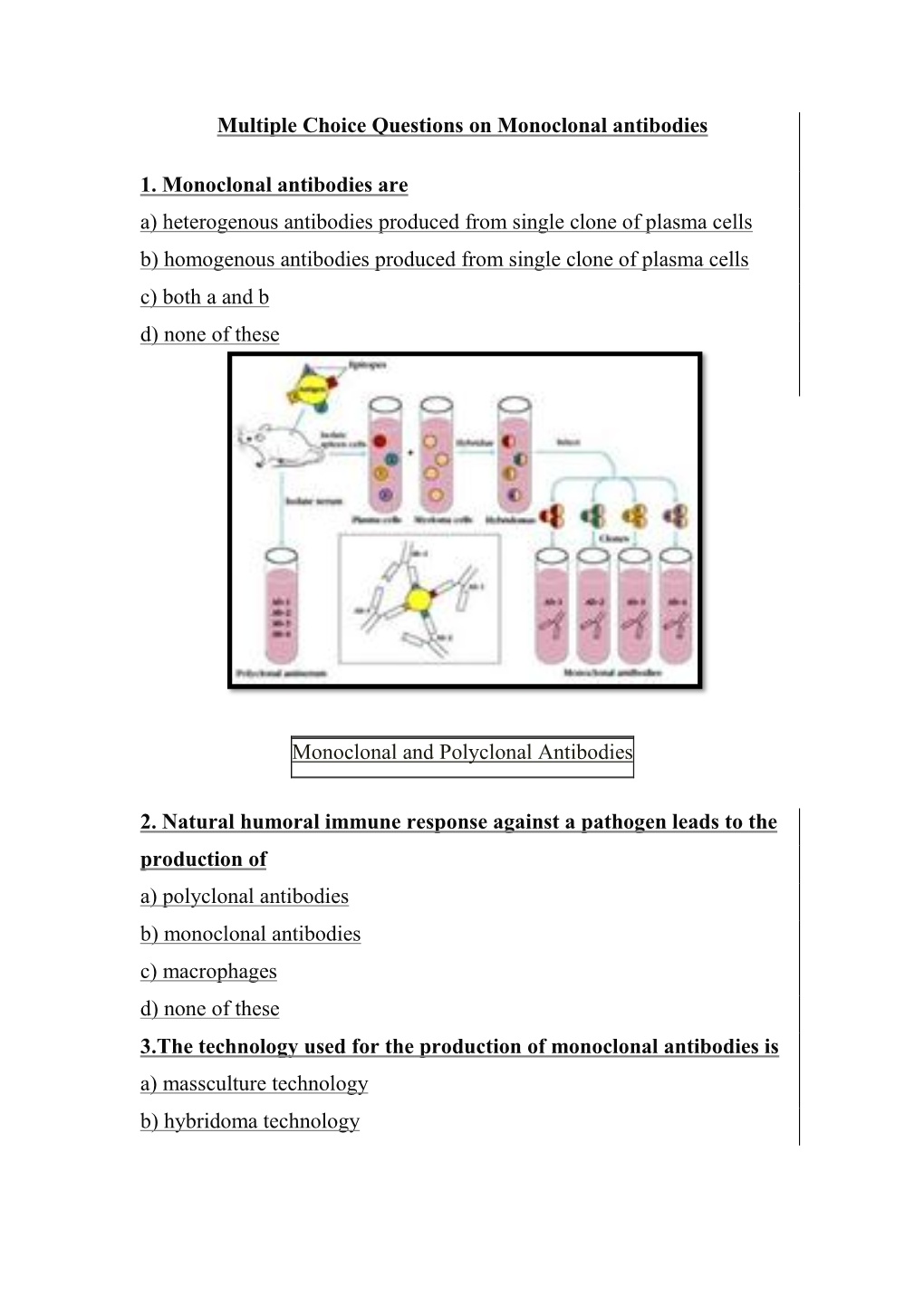
Load more
Recommended publications
-

Hybridoma Technology History of Hybridoma Technology
Hybridoma Technology History of Hybridoma Technology What is hybridoma technology? Hybridoma technology is a well-established method to produce monoclonal antibodies (mAbs) specific to antigens of interest. Hybridoma cell lines are formed via fusion between a short-lived antibody-producing B cell and an immortal myeloma cell. Each hybridoma constitutively expresses a large amount of one specific mAb, and favored hybridoma cell lines can be cryopreserved for long-lasting mAb production. As a result, researchers usually prefer generating hybridomas over other mAb production methods in order to maintain a convenient, never-ending supply of important mAbs. Fig 1. Hybridoma technology Inventor: Georges Kohler and Cesar Milstein Hybridoma technology was discovered in 1975 by two scientists, Georges Kohler and Cesar Milstein. They wanted to create immortal hybrid cells by fusing normal B cells from immunized mice with their myeloma cells. For incidental reasons, they had all the requirements fulfilled and it worked in the first attempt. By cloning individual hybrid cells, they established the first hybridoma cell lines which can produce single type of antibody specific to the specific antigen. Their discovery is considered one of the greatest breakthroughs in the field of biotechnology. For the past decades, hybridomas have fueled the discovery and production of antibodies for a multitude of applications. By utilizing hybridoma technology, Sino Biological provides cost-effective mouse monoclonal antibody service, and we can deliver you purified antibodies in 60 days. Steps Involved in Hybridoma Technology Hybridoma technology is composed of several technical procedures, including antigen preparation, animal immunization, cell fusion, hybridoma screening and subcloning, as well as characterization and production of specific antibodies. -

HAT Medium Supplement 50X, Liquid
HAT Medium supplement 50X, Liquid w/ 680.5 mg/litre Hypoxanthine, 8.8 mg/litre Aminopterin and 193.8 mg/litre Thymidine in Phosphate Buffered Saline Sterile filtered Cell Culture Tested Product Code: TCL072 Product description: Directions for use: Monoclonal antibodies are produced by hybridoma 1. Aseptically transfer 10 ml of the 50X HAT medium technology in which a non-secreting myeloma cell line is supplement to 500ml of sterile medium. fused with an antibody-producing B-lymphocyte. After 2. Tightly cap the media bottle and mix gently to ensure fusion, the proportion of viable hybrids is low. Hence, proper mixing. selective media are required to favor the survival of Note: Do not mix vigorously as it may lead to hybrids at the expense of the parental cells. Hybrids are formation of foam. most frequently selected with the HAT system. 3. The final concentrations of hypoxanthine, aminopterin and thymidine in 500ml media will be HAT supplement contains Hypoxanthine, Aminopterin 100mM, 0.4mM and 16mM respectively. and Thymidine and is used for the preparation of selection medium for hybridoma. The myeloma cell is deficient in enzyme hypoxanthine-guanine phosphoribosyl transferase (HGPRT) or thymidine kinase (TK), and cannot survive in Quality Control: selection medium containing hypoxanthine, aminopterin Appearance and thymidine. Any unfused B-lymphocytes from the Colorless, clear solution spleen cannot survive in culture for more than a few days. pH It is essential for B-cell-myeloma hybrids to contain the 8.90 to 9.50 genetic information from both parent cells to enable them Cell Culture Test to survive in the HAT selection system. -

Dynamics of Pyrimidine Deoxynucleoside Triphosphate
Proc. Nati Acad. Sci. USA Vol. 80, pp. 1347-1351, March 1983 Cell Biology Dynamics of pyrimidine deoxynucleoside triphosphate pools in relationship to DNA synthesis in 3T6 mouse fibroblasts (nucleoside incorporation/compartmentation/amethopterin block) BjORN NICANDER AND PETER REICHARD Medical Nobel Institute, Department of Biochemistry I, Karolinska Institutet, Box 60400, S-104 01 Stockholm, Sweden Contributed by Peter A. Reichard, November 18, 1982 ABSTRACT The 3H-labeled nucleosides cytidine, deoxycyti- tivity into DNA to this value (1, 2). Such a procedure becomes dine, and thymidine are rapidly incorporated into DNA via dCTP imperative when one wishes to distinguish between effects of or dTTP pools. Between 30 and 60 minafter addition of tracer different manipulations-e. g., pharmacological interference- amounts ofa labeled nucleoside to the medium ofrapidly growing on precursor synthesis and DNA replication. Knowing the spe- 3T6 cells, dNTPpools attained a constant specific activity resulting cific activity of a dNTP under steady-state conditions may in from a steady-state equilibrium between incorporation of nucleo- addition permit determination of absolute rather than relative side, de novo synthesis, and linear incorporation of isotope into rates of DNA synthesis. DNA. Removal oflabeled deoxycytidine or thymidine depleted the The of dNTP pools ofisotope within a few minutes and incorporation into possibility compartmentation of dNTP pools poses DNA stopped. When de novo synthesis ofdTTP was blocked with additionalicomplications (3). Fractionation ofcells in nonaque- amethopterin, the intracellular dTTP pool rapidly reached the ous media led to -the suggestion of separate cytoplasmic and. specific activity of thymidine of' the medium and isotope-incor- nuclear dNTP pools in Chinese hamster ovary cells (4). -

Perspectives on in Vitro Diagnostic Devices, Regulated by the Office Of
1 2 “This transcript appears as received from the commercial transcribing service after inclusion of minor corrections to typographical and factual errors recommended by the Technical Point of Contact.” 1 PERSPECTIVES ON IN VITRO DIAGNOSTIC DEVICES, 2 REGULATED BY THE OFFICE OF BLOOD RESEARCH AND REVIEW 3 4 FOOD AND DRUG ADMINISTRATION 5 WHITE OAK CAMPUS 6 BUILDING 31 7 10903 NEW HAMPSHIRE AVENUE 8 SILVER SPRING, MARYLAND 20903 9 10 TUESDAY, JULY 16, 2019 11 8:30 A.M. 12 13 APPEARANCES: 14 MODERATOR: TERESITA C. MERCADO, MS 15 16 WELCOME: 17 JULIA LATHROP, PHD, DETTD/OBRR 18 19 INTRODUCTION: 20 WENDY PAUL, MD, DBCD/OBRR 21 22 PRESENTERS: 23 KIMBERLY BIGLER, MLS(ASCP)CMSBB, DBCD/OBRR 1 ANNETTE RAGOSTA, MT(ASCP)SBB, DBCD/OBRR 2 ZHUGONG "JASON" LIU, PHD, DRB/DBCD 3 4 CONFERENCE 5 TUESDAY, JULY 16, 2019 6 8:30 A.M. 7 DR. LATHROP: Okay, everybody. Thank you for coming to the second day of 8 our workshop which is going to be focused on considerations for IVDs regulated by the Division 9 of Blood Components and Devices in OBRR. 10 Again, the slides will be available on the website in about two weeks, so take a 11 look for them there. And moderating today’s session is Teresita Mercado from the Division. 12 MS. MERCADO: Good morning. Welcome to session three of the IVD 13 workshop. Our first speaker will be Dr. Wendy Paul. She is the deputy director of DBCD who 14 will give us an introduction to devices in DBCD. 15 DR. -

Hybridoma Technology
HYBRIDOMA TECHNOLOGY Subject : Dr. KRISHNA KUMAR V M.sc,Ph.D,PGDCS Govt. Degree College ,NAIDUPET Email. Id : [email protected] HYBRIDOMA TECHNOLOGY LEARNING OBJECTIVES • TO UNDERSTAND THE NATURE OF ANTIBODY • DISTINGUISH BETWEEN POLYCLONAL AND MONOCLONAL ANTIBODIES • TO LEARN THE TECHNIQUE INVOLVED IN HYBRIDOMA TECHNOLOGY • STEPS INVOLVED IN I) CELL- FUSION III) ANTIBODY PRODUCTION INTRODUCTION ANTIBODY : • Antibodies are specialised proteins that are recruited by the immune system in response to an antigenic stimulus. • Antibodies identify and recognise the foreign antigens and remove them from the body. • Antibodies are produced B lymphocytes (or) B-cells • The production of antibodies is the main function of the humoral response (or) adaptive Immune system TYPES OF ANTIBODIES POLYCLONAL ANTIBODIES: • Antibodies derived from different B lymphocyte cell lines. • Heterogeneous in nature collected from multiple B cell clones. • Recognise and bind to many different epitopes of single antigen. • Primary role is to detect unknown antigens. Monoclonal antibody MONOCLONAL ANTIBODIES (Mabs) : • Antibodies are identical as they are produced by one type of immune cell (or) Clones of single parent cell. • Homogeneous in nature with affinity towards a specific antigen MONOCLONAL ANTIBODIES (Mabs) • Monoclonal antibodies possess two important characteristics, namely high specificity and high reproducibility. • widely used in diagnostic, clinical and therapeutic applications. • Plays a key role in immunotherapy HYBRIDOMA TECHNOLOGY MONOCLONAL ANTIBODIES : • Production of monoclonal antibodies using hybridoma technology was discovered in 1975 by GEORGE KOHLER & CESAR MILSTEIN. • They shared the NOBEL PRIZE in the year,1984. nobelprize.org • In 1990 MILSTEIN produced the first monoclonal antibodies HYBRIDOMA TECHNOLOGY HYBRIDOMA TECHNIQUE : • Well established method to produce monoclonal antibodies specific to desired antigens. -

Producing a Peptide for Use in a Blood Biosensor for Injury
Producing A Peptide For Use In A Blood Biosensor For Injury Detection By Errek Mạnh Trung Phạm Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Biological Sciences Program YOUNGSTOWN STATE UNIVERSITY December 2020 Producing A Peptide For Use In A Blood Biosensor For Injury Detection Errek Mạnh Trung Phạm I hereby release this thesis to the public. I understand that this thesis will be made available from the OhioLINK ETD Center and the Maag Library Circulation Desk for public access. I also authorize the University or other individuals to make copies of this thesis as needed for scholarly research. Signature: ____________________________________________________________ Errek M.T. Phạm, Master’s Student Date Approvals: ____________________________________________________________ Dr. Diana L. Fagan, Thesis Advisor Date ____________________________________________________________ Dr. Jonathan J. Caguiat, Committee Member Date ____________________________________________________________ Dr. David K. Asch, Committee Member Date ____________________________________________________________ Dr. Salvatore A. Sanders, Dean of Graduate Studies Date ii Abstract Conventional hybridoma technology has been used for the selection and production of proteins for biosensor development. However, hybridoma technology is typically a slower and more costly procedure than phage display and produces a less durable end- product. Quicker and more efficient production of a small peptide using phage display has been -

BIO 02 - Production of a Monoclonal Antibody Against SARS-Cov-2 and Determination of Viral Neutralization Capacity
BIO_02 - Production of a monoclonal antibody against SARS-CoV-2 and determination of viral neutralization capacity Nathalie Bonatti Franco Almeida1*; Camila Amormino Corsini1; Priscilla Soares Filgueiras1; Daniel Alvim Pena de Miranda1; Lucélia Antunes Coutinho1; Patrícia Martins Parreiras1; Rafaella Fortini Grenfel e Queiroz1. 1Fiocruz/CPqRR. Introduction: The emergence of the new coronavirus (SARS-CoV-2) in Wuhan, China, caused a worldwide epidemic of respiratory disease (COVID-19). SARS-CoV-2 belongs to the genus Betacoronavirus. It has structural proteins that include the spike protein (S), the envelope protein (E), the membrane protein (M) and the nucleocapsid protein (N). Various reports were published relating the antibody responses generated against protein S, as it is the most exposed protein of SARS-CoV-2. Monoclonal antibodies (mAbs) have many applications in diagnosis, treatment and can contribute to study the COVID-19. Objective: Production of a monoclonal antibodies against SARS-CoV-2 spike protein and determination of the neutralization of SARS-CoV-2 infection. Methodology: 1. Mouse Immunizations.SARS-CoV-2 spike protein (GenBank: MN908947) was mixed with an equivalent volume of Vaccine Self-Assembling Immune Matrix (VacSIM) adjuvant and injected subcutaneously into BALB/c mice. 40 µg of protein was used in the first injection and 20 µg at 9, 23, 30, 36, 52, 58, 64 days after the first injection. Spike protein without adjuvant was injected intraperitoneally 3 days prior to removal of the mouse spleen and cell fusion. 2. Determination of antibody titer against Spike protein.To determine the antibody titer against the SARS-CoV-2 spike protein, at 6 days after each immunization, the serum from mice was tested by ELISA. -

Monoclonal Antibodies As Tools to Combat Fungal Infections
Journal of Fungi Review Monoclonal Antibodies as Tools to Combat Fungal Infections Sebastian Ulrich and Frank Ebel * Institute for Infectious Diseases and Zoonoses, Faculty of Veterinary Medicine, Ludwig-Maximilians-University, D-80539 Munich, Germany; [email protected] * Correspondence: [email protected] Received: 26 November 2019; Accepted: 31 January 2020; Published: 4 February 2020 Abstract: Antibodies represent an important element in the adaptive immune response and a major tool to eliminate microbial pathogens. For many bacterial and viral infections, efficient vaccines exist, but not for fungal pathogens. For a long time, antibodies have been assumed to be of minor importance for a successful clearance of fungal infections; however this perception has been challenged by a large number of studies over the last three decades. In this review, we focus on the potential therapeutic and prophylactic use of monoclonal antibodies. Since systemic mycoses normally occur in severely immunocompromised patients, a passive immunization using monoclonal antibodies is a promising approach to directly attack the fungal pathogen and/or to activate and strengthen the residual antifungal immune response in these patients. Keywords: monoclonal antibodies; invasive fungal infections; therapy; prophylaxis; opsonization 1. Introduction Fungal pathogens represent a major threat for immunocompromised individuals [1]. Mortality rates associated with deep mycoses are generally high, reflecting shortcomings in diagnostics as well as limited and often insufficient treatment options. Apart from the development of novel antifungal agents, it is a promising approach to activate antimicrobial mechanisms employed by the immune system to eliminate microbial intruders. Antibodies represent a major tool to mark and combat microbes. Moreover, monoclonal antibodies (mAbs) are highly specific reagents that opened new avenues for the treatment of cancer and other diseases. -

Hybridoma Technology & Monoclonal Antibody Production Definition
Sanjib Kr Das B.Sc Hons. In ZOOLOGY 06.06.2020 Assistant Professor (WBES) SEM-IV Dept. of Zoology Paper- CC-10 Immunology Jhargram Raj College UNIT-4 Immunoglobulin Hybridoma Technology & Monoclonal Antibody Production Definition: Hybridoma A clone of hybrid cells formed by fusion of normal lymphocytes with myeloma cells. It retains the properties of the normal cell to produce antibodies or T-cell receptors but exhibits the immortal growth characteristic of myeloma cells. Hybridomas are used to produce monoclonal antibody (mAB). Monoclonal antibody Homogeneous preparation of antibody molecules, produced by a single clone of B lineage cells, often a hybridoma, all of which have the same antigenic specificity. (The term monoclonal refers to the fact that all of the cells in a given hybridoma culture are derived from the single clone of cells, and therefore carry the same DNA). A hybridoma is a fusion product of two cells. B-cell hybridomas are generated by artificially fusing antibody-producing, short-lived lymphocytes with long-lived tumor cells in order to generate long-lived daughter cells secreting large amounts of monoclonal antibodies. In 1975, Georges Köhler and Cesar Milstein figured out how to generate large quantities of antibodies derived from a single B-cell clone. By fusing a normal, activated, antibody-producing B cell with a myeloma cell (a cancerous plasma cell), they were able to generate a hybridoma that possessed the immortal growth properties of the myeloma cell parent and secreted the unique antibody produced by the B-cell parent. Over time, myeloma-cell partners were generated that had lost the ability to synthesize their own immunoglobulin, thus ensuring that the only antibodies secreted into the culture medium were those from the B-cell fusion partner. -

Lecture on the Topic-Hybridoma Technology
Course Code: ZOOL-2023; Course Title: Immunology Programme: B.Sc. Zoology (Hons.) Dr. Kundan Kishor Rajak Assistant Professor Deptt. of Zoology School of Life Sciences Mahatma Gandhi Central University Bihar • In hybridoma technology, monoclonal antibodies are made out side the body by hybrid cell cultures known as hybridomas. • Hybridomas are cells formed via fusion between a short-lived antibody-producing Plasma cell and an immortal myeloma cell (Cancer cell). Plasma cell Myeloma cell A naive B-lymphocyte Myeloma is a form of cancer immediately differentiate after that begins in the blood’s plasma encounters with an antigen into cells. memory B-cell and effector B- Plasma cell transformed into cells called Plasma cell. malignant cell known as Plasma cell produce secretory myeloma cell. antibody. Grow and spread It live for only a few days. uncontrollably. A single plasma cell can secrete Immortal in character. more than 2000 molecules of Myeloma cells are also produce antibody per second. secretory antibody but impaired. + = Plasma cell Myeloma cell Hybridoma cell In 1973, Jeriod Schwaber and Ed Cohen (from Liribida institute, University of Chicago) – First to produce hybridoma cell through fusion of human B-lymphocyte cells and myeloma cells . In 1975, Georges Kohler and Cesar Milstein (from Medical Research Council Laboratory, Cambridge, UK)- construct a continuous cell lines, which secrete monoclonal antibodies of a desired specificity. In 1984, Kohler and Milstein awarded Nobel Prize jointly with Niels Jerne in Physiology and Medicine. • The hybridoma technique involves the fusion of plasma cells (Effector B-Lymphocyte cells) harvested from spleen of mice, already immunized with immunogen, with a myeloma cell lines, which is capable of grow in animal cell culture medium. -

Antibody Drug Development: Challenges & Solutions
Antibody Drug Development: Challenges & Solutions Liusong Yin, Ph.D. [email protected] Table of Contents 1 ADD overview 2 Platform & technologies 3 Anti-idiotype antibody 4 Case studies 5 Ab services specifications Make Research Easy 2 Antibody Drug Market Gary Walsh, 2014, Nature Biotechnology Make Research Easy 3 Antibody Drug Development Process TargetTarget Antigen Immunization Functional Lead Antibody Preclinical SelectionValidation Production & Hybridoma Assay Optimization Production Candidate Make Research Easy 4 Technologies to Generate Therapeutic Antibodies Hybridoma with human transgenic mice >>>> Human antibody Hybridoma with B-cells from immunized human body >>>> Human antibody Hybridoma with rodent system >>>> Humanized antibody SLAM technology >>>> Humanized antibody Phage/yeast display >>>> Human/Humanized antibody Next generation antibody sequence >>>> Human/Humanized antibody Make Research Easy 5 Hybridoma Technology – Old but Powerful • By 2015, FDA has approved 44 therapeutic antibody drugs. • 41 out of 44 were derived from hybridoma technology, 3 from phage-display technology. • The hybridoma technology generates high market value. Make Research Easy 6 Hybridoma - The Critical Path for Ab Lead Identification Reagent Animal Assay preparation Immunization Development Hybridoma library: 1-2x104 clones in 25 plates/ fusion, total 4-8 fusions o 1 . In vitro target binding assay (selectivity against family members) o 2 . Cell based blocking assay o 3 . Cell based functional assay Hit subcloning and characterization -

Hybridoma Technology: the Preferred Method for Monoclonal Antibody
ReportsExpert Opinion 2019FIRST DRAFT SUBMITTED: 29 04 2019; Hybridoma technology: the preferred method for ACCEPTED FOR PUBLI- CATION: 5 monoclonal antibody generation for in vivo applications 0 7 2019; Samantha Zaroff*,1 & Grace Tan1 PUBLISHED ONLINE: 00 00 0000 Prior to the development of hybridoma mAbs. In brief, the first stage involves technology, researchers relied on polyclonal the development and optimization of an antibodies (pAbs) for experiments involving immunogenic antigen (Ag) [3]. Next, a the identification and quantification of host animal is immunized with the Ag to specific proteins of interest within complex elicit an immune response and initiate biological environments. While pAbs the process of B-cell maturation [2,4]. The offered their own set of benefits, they could third stage involves the isolation of these B not be used for in vivo experiments or thera- cells from the spleen of the host animal and peutics due to their batch-to-batch incon- their fusion with myeloma cells to generate sistency and high levels of background hybridomas [5]. During the fourth stage, reactivity. It was not until 1975 when Köhler the generated hybridomas are subject to and Milstein used hybridomas to generate multiple rounds of screening and selection monoclonal antibodies (mAbs), that the in order to identify the hybridomas that worlds of antibodies and in vivo scientific produce the best mAbs for the intended research could meet for the first time [1,2]. downstream application. The fifth and The introduction of mAbs to in vivo research final stage is the amplification of these With ADCs and gave rise to some of the greatest scientific specific hybridomas and subsequent mAb © 2019 SAMANTHA ZAROFF targeted“ biologics achievements of the 21st century, including purification [3].