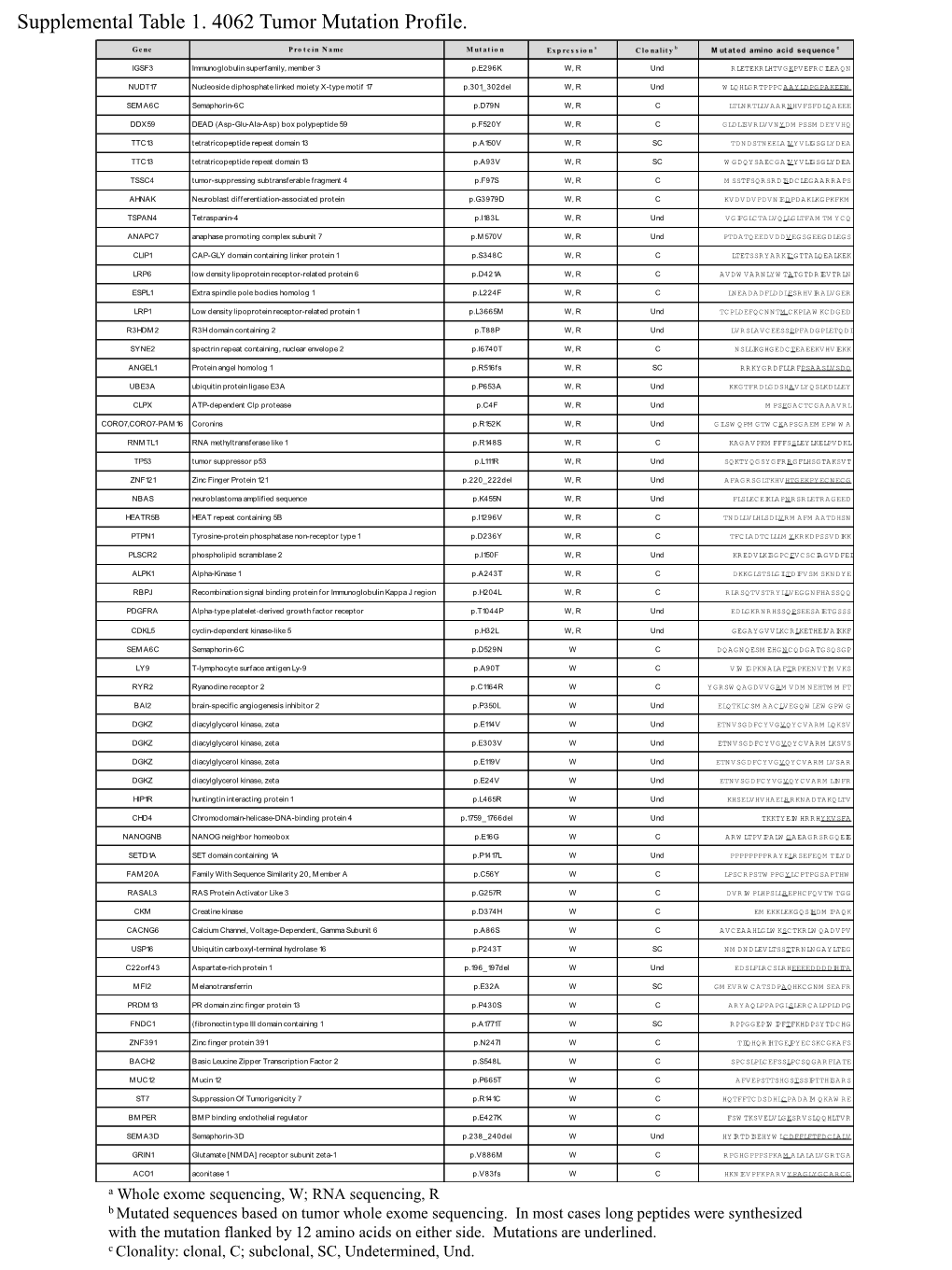
Supplemental Table 1. 4062 Tumor Mutation Profile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

METACYC ID Description A0AR23 GO:0004842 (Ubiquitin-Protein Ligase
Electronic Supplementary Material (ESI) for Integrative Biology This journal is © The Royal Society of Chemistry 2012 Heat Stress Responsive Zostera marina Genes, Southern Population (α=0. -

I HIGH MASS ACCURACY COUPLED to SPATIALLY-DIRECTED
HIGH MASS ACCURACY COUPLED TO SPATIALLY-DIRECTED PROTEOMICS FOR IMPROVED PROTEIN IDENTIFICATIONS IN IMAGING MASS SPECTROMETRY EXPERIMENTS By David Geoffrey Rizzo Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Chemistry August, 2016 Nashville, Tennessee Approved: Richard M. Caprioli, Ph.D. Kevin L. Schey, Ph.D. John A. McLean, Ph.D. Michael P. Stone, Ph.D. i Copyright © 2016 by David Geoffrey Rizzo All Rights Reserved ii This work is dedicated to my family and friends, who have shown nothing but support for me in all of life’s endeavors. iii ACKNOWLEDGEMENTS “As we express our gratitude, we must never forget that the highest appreciation is not to utter words, but to live by them.” - John F. Kennedy – There are many people I must thank for showing kindness, encouragement, and support for me during my tenure as a graduate student. First and foremost, I would like to thank my research advisor, Richard Caprioli, for providing both ample resources and guidance that allowed me to grow as a scientist. Our discussions about my research and science in general have helped me become a much more focused and discerning analytical chemist. I must also thank my Ph.D. committee members, Drs. Kevin Schey, John McLean, and Michael Stone, who have brought valuable insight into my research and provided direction along the way. My undergraduate advisor, Dr. Facundo Fernández, encouraged me to begin research in his lab and introduced me to the world of mass spectrometry. -

Constitutive Phospholipid Scramblase Activity of a G Protein-Coupled Receptor
ARTICLE Received 19 Feb 2014 | Accepted 1 Sep 2014 | Published 8 Oct 2014 DOI: 10.1038/ncomms6115 Constitutive phospholipid scramblase activity of a G protein-coupled receptor Michael A. Goren1, Takefumi Morizumi2, Indu Menon1, Jeremiah S. Joseph3,w, Jeremy S. Dittman1, Vadim Cherezov3, Raymond C. Stevens3, Oliver P. Ernst2,4 & Anant K. Menon1 Opsin, the rhodopsin apoprotein, was recently shown to be an ATP-independent flippase (or scramblase) that equilibrates phospholipids across photoreceptor disc membranes in mammalian retina, a process required for disc homoeostasis. Here we show that scrambling is a constitutive activity of rhodopsin, distinct from its light-sensing function. Upon reconstitution into vesicles, discrete conformational states of the protein (rhodopsin, a metarhodopsin II-mimic, and two forms of opsin) facilitated rapid (410,000 phospholipids per protein per second) scrambling of phospholipid probes. Our results indicate that the large conformational changes involved in converting rhodopsin to metarhodopsin II are not required for scrambling, and that the lipid translocation pathway either lies near the protein surface or involves membrane packing defects in the vicinity of the protein. In addition, we demonstrate that b2-adrenergic and adenosine A2A receptors scramble lipids, suggesting that rhodopsin-like G protein-coupled receptors may play an unexpected moonlighting role in re-modelling cell membranes. 1 Department of Biochemistry, Weill Cornell Medical College, New York, New York 10065, USA. 2 Department of Biochemistry, University of Toronto, Toronto, Ontario, Canada M5S 1A8. 3 Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, California 92037, USA. 4 Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada M5S 1A8. -

Phospholipid Ebb and Flow Makes Mitochondria Go
REVIEW Phospholipid ebb and flow makes mitochondria go Michelle Grace Acoba, Nanami Senoo, and Steven M. Claypool Mitochondria, so much more than just being energy factories, also have the capacity to synthesize macromolecules including phospholipids, particularly cardiolipin (CL) and phosphatidylethanolamine (PE). Phospholipids are vital constituents of mitochondrial membranes, impacting the plethora of functions performed by this organelle. Hence, the orchestrated movement of phospholipids to and from the mitochondrion is essential for cellular integrity. In this review, we capture recent advances in the field of mitochondrial phospholipid biosynthesis and trafficking, highlighting the significance of interorganellar communication, intramitochondrial contact sites, and lipid transfer proteins in maintaining membrane homeostasis. We then discuss the physiological functions of CL and PE, specifically how they associate with protein complexes in mitochondrial membranes to support bioenergetics and maintain mitochondrial architecture. Introduction (PS), phosphatidylinositol (PI), and phosphatidylcholine (PC), as Biological membranes give a means of regulated communi- well as sphingolipids, cholesterol, and many lysophospholipids, it cation, as the lipid bilayer itself acts as a platform for many has to recruit from other organelles. This section will focus on the reactions. The amphipathic nature of lipids underlies the mitochondrion’s contribution to the generation of two highly creation of a bilayer; the hydrophobic acyl chains cluster at abundant phospholipids in its membranes: CL and PE (Fig. 1). the middle to get buried, while the hydrophilic head groups face the aqueous surroundings. A diverse array of lipids, in- CL metabolism cluding glycerophospholipids, sphingolipids, and sterols, present CL production. CL is the defining membrane constituent of in different amounts, gives a biological membrane its identity the mitochondrion, the organelle in which it is made (Fig. -

Phosphatidylserine in Non-Apoptotic Cell Death Inbar Shlomovitz1, Mary Speir2,3 and Motti Gerlic1*
Shlomovitz et al. Cell Communication and Signaling (2019) 17:139 https://doi.org/10.1186/s12964-019-0437-0 REVIEW Open Access Flipping the dogma – phosphatidylserine in non-apoptotic cell death Inbar Shlomovitz1, Mary Speir2,3 and Motti Gerlic1* Abstract The exposure of phosphatidylserine (PS) on the outer plasma membrane has long been considered a unique feature of apoptotic cells. Together with other “eat me” signals, it enables the recognition and phagocytosis of dying cells (efferocytosis), helping to explain the immunologically-silent nature of apoptosis. Recently, however, PS exposure has also been reported in non-apoptotic forms of regulated inflammatory cell death, such as necroptosis, challenging previous dogma. In this review, we outline the evidence for PS exposure in non-apoptotic cells and extracellular vesicles (EVs), and discuss possible mechanisms based on our knowledge of apoptotic-PS exposure. In addition, we examine the outcomes of non-apoptotic PS exposure, including the reversibility of cell death, efferocytosis, and consequent inflammation. By examining PS biology, we challenge the established approach of distinguishing apoptosis from other cell death pathways by AnnexinV staining of PS externalization. Finally, we re- evaluate how PS exposure is thought to define apoptosis as an immunologically silent process distinct from other non-apoptotic and inflammatory cell death pathways. Ultimately, we suggest that a complete understanding of how regulated cell death processes affect the immune system is far from being fully -

Negative Regulation of Diacylglycerol Kinase &Theta
Cell Death and Differentiation (2010) 17, 1059–1068 & 2010 Macmillan Publishers Limited All rights reserved 1350-9047/10 $32.00 www.nature.com/cdd Negative regulation of diacylglycerol kinase h mediates adenosine-dependent hepatocyte preconditioning G Baldanzi1,5, E Alchera2,5, C Imarisio2, M Gaggianesi1, C Dal Ponte2, M Nitti3, C Domenicotti3, WJ van Blitterswijk4, E Albano2, A Graziani1,5 and R Carini*,2,5 In liver ischemic preconditioning (IP), stimulation of adenosine A2a receptors (A2aR) prevents ischemia/reperfusion injury by promoting diacylglycerol-mediated activation of protein kinase C (PKC). By concerting diacylglycerol to phosphatidic acid, diacylglycerol kinases (DGKs) act as terminator of diacylglycerol signalling. This study investigates the role of DGK in the development of hepatocyte IP. DGK activity and cell viability were evaluated in isolated rat hepatocytes preconditioned by 10 min hypoxia followed by 10 min re-oxygenation or by the treatment with the A2aR agonist, CGS21680, and subsequently exposed to prolonged hypoxia. We observed that after IP or A2aR activation, a decrease in DGK activity was associated with the onset of hepatocyte tolerance to hypoxia. CGS21680-induced stimulation of A2aR specifically inhibited DGK isoform h by activating RhoA–GTPase. Consistently, both siRNA-mediated downregulation of DGK h and hepatocyte pretreatment with the DGK inhibitor R59949 induced cell tolerance to hypoxia. The pharmacological inhibition of DGK was associated with the diacylglycerol- dependent activation of PKC d and e and of their downstream target p38 MAPK. In conclusion, we unveil a novel signalling pathway contributing to the onset of hepatocyte preconditioning, which through RhoA–GTPase, couples A2aR to the downregulation of DGK. -

Phosphatidylserine, a Death Knell
Cell Death and Differentiation (2001) 8, 551 ± 563 ã 2001 Nature Publishing Group All rights reserved 1350-9047/01 $15.00 www.nature.com/cdd REVIEW Phosphatidylserine, a death knell# RA Schlegel*,1 and P Williamson2 molecules are sometimes withdrawn from the matrix and hydrolyzed to produce signaling molecules, including 1 Department of Biochemistry and Molecular Biology, Penn State University, prostaglandins, diacylglycerol and ceramides. In recent University Park, PA 16802, USA years, however, a growing body of evidence has suggested 2 Department of Biology, Amherst College, Amherst, MA 01002, USA that physical and chemical properties of the bilayer itself, such * Corresponding author: RA Schlegel, Department of Biochemistry and as the thickness of the hydrophobic core1 or local lateral Molecular Biology, 428 South Frear Laboratory, Penn State University, 2±4 University Park, PA 16802, USA. Tel: (814) 865-6974; Fax: (814) 863-7024; domains of specialized lipid composition may play E-mail: [email protected] significant roles in the assembly and organization of cellular membranes. In addition to these structural contributions to Received 30.8.00; revised 13.11.00; accepted 27.11.00 membrane function, the past few years have also seen the Edited by M Piacentini revelation that a phospholipid itself, and not a derived product, acts on the extracytosolic, external face of the plasma membrane to regulate intercellular interactions. Abstract Appreciation of this new role for phospholipids was Virtually every cell in the body restricts phosphatidylserine galvanized by the demonstration that phosphatidylserine (PS) to the inner leaflet of the plasma membrane by energy- (PS) appears on the surface of apoptotic lymphocytes and dependent transport from the outer to the inner leaflet of the contributes to their phagocytosis by activated macro- phages.5 The functional importance of the phagocytosis of bilayer. -

Domains, Amino Acid Residues, and New Isoforms of Caenorhabditis Elegans Diacylglycerol Kinase 1 (DGK-1) Important for Terminating Diacylglycerol Signaling in Vivo*□S
Supplemental Material can be found at: http://www.jbc.org/content/suppl/2004/12/06/M409460200.DC1.html THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 280, No. 4, Issue of January 28, pp. 2730–2736, 2005 © 2005 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Domains, Amino Acid Residues, and New Isoforms of Caenorhabditis elegans Diacylglycerol Kinase 1 (DGK-1) Important for Terminating Diacylglycerol Signaling in Vivo*□S Received for publication, August 17, 2004, and in revised form, November 22, 2004 Published, JBC Papers in Press, November 24, 2004, DOI 10.1074/jbc.M409460200 Antony M. Jose‡ and Michael R. Koelle§¶ From the ‡Departments of Molecular, Cellular, and Developmental Biology and §Molecular Biophysics and Biochemistry, Yale University School of Medicine, New Haven, Connecticut 06520 Diacylglycerol kinases (DGKs) inhibit diacylglycerol numerous cellular processes mediated by neurotransmitters, (DAG) signaling by phosphorylating DAG. DGK-1, the growth factors, and hormones (3). DAG also activates the syn- Caenorhabditis elegans ortholog of human neuronal aptic vesicle priming protein UNC-13 (4, 5) to control neuro- DGK, inhibits neurotransmission to control behavior. transmission and certain transient receptor potential cation Downloaded from DGK-1, like DGK, has three cysteine-rich domains channels (6). In humans, nine DGK isozymes have been iden- (CRDs), a pleckstrin homology domain, and a kinase tified (DGK␣, , ␥, ␦, , ⑀, , , and ), but their physiological domain. To identify DGK domains and amino acid resi- functions remain largely unknown (1, 2). dues critical for terminating DAG signaling in vivo,we C. elegans DGK-1 provides a genetically tractable model for analyzed 20 dgk-1 mutants defective in DGK-1-con- elucidating the physiological functions of diacylglycerol ki- trolled behaviors. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Transcriptomic and Proteomic Profiling Provides Insight Into
BASIC RESEARCH www.jasn.org Transcriptomic and Proteomic Profiling Provides Insight into Mesangial Cell Function in IgA Nephropathy † † ‡ Peidi Liu,* Emelie Lassén,* Viji Nair, Celine C. Berthier, Miyuki Suguro, Carina Sihlbom,§ † | † Matthias Kretzler, Christer Betsholtz, ¶ Börje Haraldsson,* Wenjun Ju, Kerstin Ebefors,* and Jenny Nyström* *Department of Physiology, Institute of Neuroscience and Physiology, §Proteomics Core Facility at University of Gothenburg, University of Gothenburg, Gothenburg, Sweden; †Division of Nephrology, Department of Internal Medicine and Department of Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, Michigan; ‡Division of Molecular Medicine, Aichi Cancer Center Research Institute, Nagoya, Japan; |Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden; and ¶Integrated Cardio Metabolic Centre, Karolinska Institutet Novum, Huddinge, Sweden ABSTRACT IgA nephropathy (IgAN), the most common GN worldwide, is characterized by circulating galactose-deficient IgA (gd-IgA) that forms immune complexes. The immune complexes are deposited in the glomerular mesangium, leading to inflammation and loss of renal function, but the complete pathophysiology of the disease is not understood. Using an integrated global transcriptomic and proteomic profiling approach, we investigated the role of the mesangium in the onset and progression of IgAN. Global gene expression was investigated by microarray analysis of the glomerular compartment of renal biopsy specimens from patients with IgAN (n=19) and controls (n=22). Using curated glomerular cell type–specific genes from the published literature, we found differential expression of a much higher percentage of mesangial cell–positive standard genes than podocyte-positive standard genes in IgAN. Principal coordinate analysis of expression data revealed clear separation of patient and control samples on the basis of mesangial but not podocyte cell–positive standard genes. -

Thesis Template
An In Vivo Screen for Genes Involved in Central Nervous System Control of Obesity Identifies Diacylglycerol Kinase as a Regulator of Insulin Secretion by Irene Trinh A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of Molecular Genetics University of Toronto © Copyright by Irene Trinh, 2017. An In Vivo Screen for Genes Involved in Central Nervous System Control of Obesity Identifies Diacylglycerol Kinase as a Regulator of Insulin Secretion Irene Trinh Doctor of Philosophy Department of Molecular Genetics University of Toronto 2017 Abstract The increasing prevalence of obesity as well as its association with many chronic diseases has turned obesity into a major health concern worldwide. Obesity has many underlying environmental and genetic factors that disturb the balance between energy intake and energy expenditure, which is controlled by the central nervous system. The goal of my project is to help further our understanding of these CNS mechanisms by using the powerful tools available in Drosophila melanogaster to identify neuronal genes involved in energy homeostasis. Using the neuronal fru-Gal4 driver we performed an RNAi screen with 1748 genes and assayed for obese or lean phenotypes defined as increases or decreases in triacylglycerol (TAG) levels. After 3 rounds of screening I identified 25 hits that were reproducible and confirmed these phenotypes with independent RNAi lines. ii One of these hits was Diacylglycerol kinase (Dgk) whose mammalian homologues have been implicated in genome-wide association studies for metabolic defects. Manipulation of neuronal Dgk levels affects TAG and carbohydrate levels but these effects don’t seem to be mediated through Dgk’s regulation of DAG, PA levels or PKC activity. -

Migrasome Formation Is Mediated by Assembly of Micron-Scale Tetraspanin Macrodomains
ARTICLES https://doi.org/10.1038/s41556-019-0367-5 Corrected: Publisher Correction Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains Yuwei Huang1,2,6, Ben Zucker3,6, Shaojin Zhang1,2,6, Sharon Elias3, Yun Zhu4, Hui Chen5, Tianlun Ding1,2, Ying Li1,2, Yujie Sun4, Jizhong Lou5, Michael M. Kozlov 3* and Li Yu 1,2* Migrasomes are recently discovered cellular organelles that form as large vesicle-like structures on retraction fibres of migrat- ing cells. While the process of migrasome formation has been described before, the molecular mechanism underlying migra- some biogenesis remains unclear. Here, we propose that the mechanism of migrasome formation consists of the assembly of tetraspanin- and cholesterol-enriched membrane microdomains into micron-scale macrodomains, which swell into migra- somes. The major finding underlying the mechanism is that tetraspanins and cholesterol are necessary and sufficient for migra- some formation. We demonstrate the necessity of tetraspanins and cholesterol via live-cell experiments, and their sufficiency by generating migrasome-like structures in reconstituted membrane systems. We substantiate the mechanism by a theoretical model proposing that the key factor driving migrasome formation is the elevated membrane stiffness of the tetraspanin- and cholesterol-enriched macrodomains. Finally, the theoretical model was quantitatively validated by experimental demonstration of the membrane-stiffening effect of tetraspanin 4 and cholesterol. igrasomes are recently discovered cellular organelles whose To study whether the TSPAN4 expression level can affect mig- formation is closely associated with cell migration. When rasome formation, we established three normal rat kidney (NRK) Mcells crawl on extracellular substrates, long membrane pro- epithelial cell lines that stably express different levels of TSPAN4 jections, referred to as retraction fibres (RFs), are pulled out of the and green fluorescent protein (GFP) (Fig.