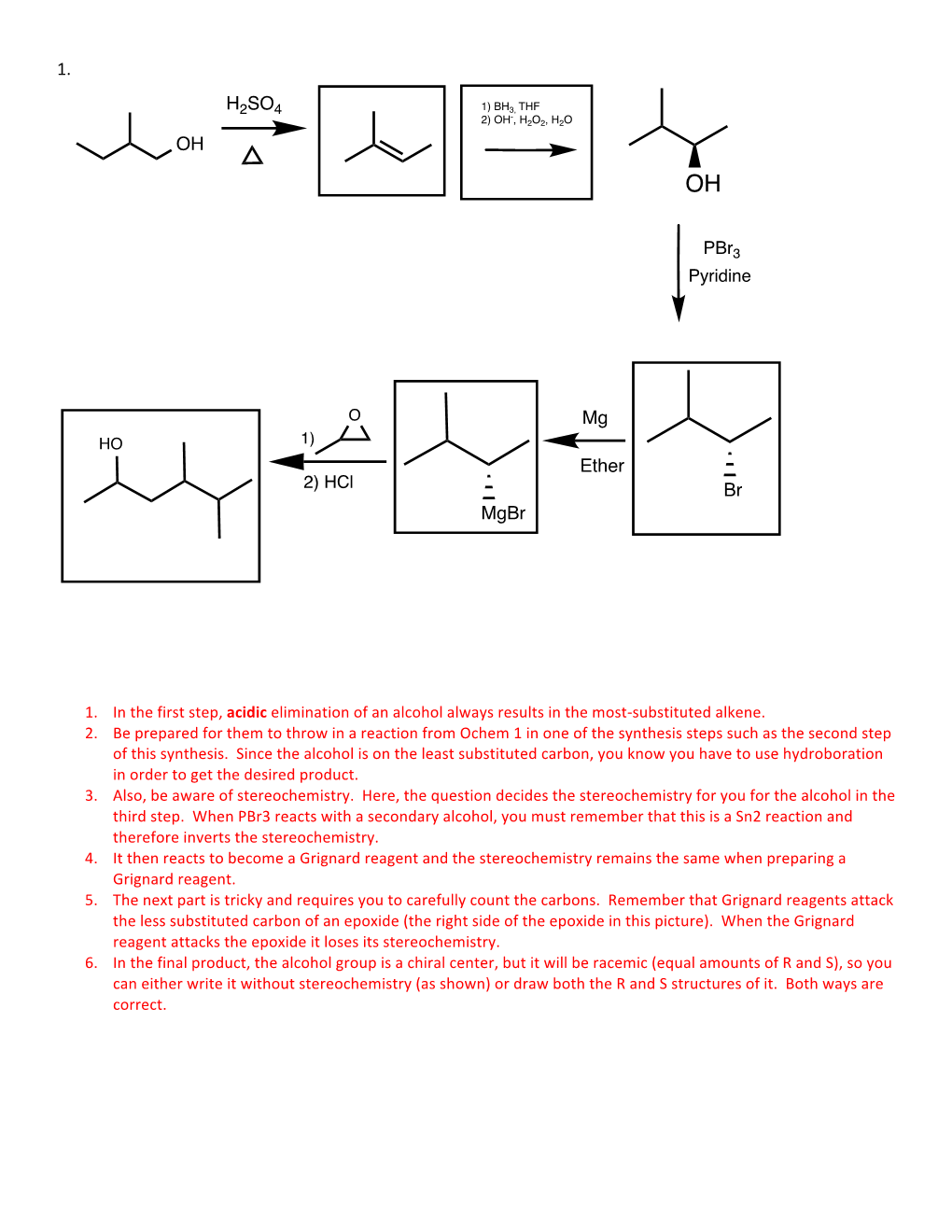
Pbr3 H2SO4 Mg Ether OH Br Mgbr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recent Advances in Titanium Radical Redox Catalysis
JOCSynopsis Cite This: J. Org. Chem. 2019, 84, 14369−14380 pubs.acs.org/joc Recent Advances in Titanium Radical Redox Catalysis Terry McCallum, Xiangyu Wu, and Song Lin* Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, United States ABSTRACT: New catalytic strategies that leverage single-electron redox events have provided chemists with useful tools for solving synthetic problems. In this context, Ti offers opportunities that are complementary to late transition metals for reaction discovery. Following foundational work on epoxide reductive functionalization, recent methodological advances have significantly expanded the repertoire of Ti radical chemistry. This Synopsis summarizes recent developments in the burgeoning area of Ti radical catalysis with a focus on innovative catalytic strategies such as radical redox-relay and dual catalysis. 1. INTRODUCTION a green chemistry perspective, the abundance and low toxicity of Ti make its complexes highly attractive as reagents and Radical-based chemistry has long been a cornerstone of 5 1 catalysts in organic synthesis. synthetic organic chemistry. The high reactivity of organic IV/III radicals has made possible myriad new reactions that cannot be A classic example of Ti -mediated reactivity is the reductive ring opening of epoxides. This process preferentially readily achieved using two-electron chemistry. However, the − high reactivity of organic radicals is a double-edged sword, as cleaves and functionalizes the more substituted C O bond, the selectivity of these fleeting intermediates can be difficult to providing complementary regioselectivity to Lewis acid control in the presence of multiple chemotypes. In addition, promoted epoxide reactions. The synthetic value of Ti redox catalysis has been highlighted by their many uses in total catalyst-controlled regio- and stereoselective reactions involv- 6−10 ing free-radical intermediates remain limited,2 and the synthesis (Scheme 1). -

Unusual Regioselectivity in the Opening of Epoxides by Carboxylic Acid Enediolates
Molecules 2008, 13, 1303-1311 manuscripts; DOI: 10.3390/molecules13061303 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.org/molecules Communication Unusual Regioselectivity in the Opening of Epoxides by Carboxylic Acid Enediolates Luis R. Domingo, Salvador Gil, Margarita Parra* and José Segura Department of Organic Chemistry, Universitat de València, Dr. Moliner 50, 46100 Burjassot, Spain. Fax +34(9)63543831; E-mails: [email protected]; [email protected]; [email protected] * Author to whom correspondence should be addressed; E-mail: [email protected] Received: 29 May 2008; in revised form: 5 June 2008 / Accepted: 6 June 2008 / Published: 9 June 2008 Abstract: Addition of carboxylic acid dianions appears to be a potential alternative to the use of aluminium enolates for nucleophilic ring opening of epoxides. These conditions require the use of a sub-stoichiometric amount of amine (10% mol) for dianion generation and the previous activation of the epoxide with LiCl. Yields are good, with high regioselectivity, but the use of styrene oxide led, unexpectedly, to a mixture resulting from the attack on both the primary and secondary carbon atoms. Generally, a low diastereoselectivity is seen on attack at the primary center, however only one diastereoisomer was obtained from attack to the secondary carbon of the styrene oxide. Keywords: Lactones, lithium chloride, nucleophilic addition, regioselectivity, diastereoselectivity. Introduction Epoxides have been recognized among the most versatile compounds in organic synthesis, not only as final products [1] but as key intermediates for further manipulations. Accordingly, new synthetic developments are continuously being published [2]. Due to its high ring strain (around 27 Kcal/mol) its ring-opening, particularly with carbon-based nucleophiles, is a highly valuable synthetic strategy Molecules 2008, 13 1304 [3]. -

Highly Efficient Epoxidation of Olefins with Hydrogen Peroxide Oxidant Using Modified Silver Polyoxometalate Catalysts
African Journal of Pure and Applied Chemistry Vol. 7(2), pp. 50-55, February 2013 Available online at http://www.academicjournals.org/AJPAC DOI: 10.5897/AJPAC12.060 ISSN 1996 - 0840 ©2013 Academic Journals Full Length Research Paper Highly efficient epoxidation of olefins with hydrogen peroxide oxidant using modified silver polyoxometalate catalysts Emmanuel Tebandeke 1*, Henry Ssekaalo 1 and Ola F. Wendt 2 1Department of Chemistry, Makerere University, P. O. Box 7062 Kampala, Uganda. 2Organic Chemistry, Department of Chemistry, Lund University, P. O. Box 124, 221 00 Lund, Sweden. Accepted 31 January, 2013 The catalytic epoxidation of olefins is an important reaction in chemical industry since epoxides are versatile and important intermediates in the synthesis of fine chemicals and pharmaceuticals. The current epoxidation procedures often use stoichiometric organic and sometimes toxic inorganic oxidants that are less favourable from an environmental and economical point of view. In contrast to such processes, catalytic epoxidation processes employing H2O2 oxidant are preferred for green processing. Herein is reported a highly efficient green process for the epoxidation of olefins using H2O2 oxidant and modified silver polyoxometalate catalysts at 65°C. The preparation and activity of the catalysts are described. The method enjoys >90% conversion and ≥99% selectivity to the epoxide for a variety of cyclic and linear olefins including terminal ones. The catalysts are easily recovered by filtration and are reusable several times. Key words: Epoxidation, olefins, hydrogen peroxide, polyoxometalate, silver catalysts. INTRODUCTION The catalytic epoxidation of olefins is very important in compared to organic peroxides and peracids (Jones, the chemical industry since epoxides are versatile and 1999; Lane and Burgess, 2003). -

Part I. the Total Synthesis Of
AN ABSTRACT OF THE THESIS OF Lester Percy Joseph Burton forthe degree of Doctor of Philosophy in Chemistry presentedon March 20, 1981. Title: Part 1 - The Total Synthesis of (±)-Cinnamodialand Related Drimane Sesquiterpenes Part 2 - Photochemical Activation ofthe Carboxyl Group Via NAcy1-2-thionothiazolidines Abstract approved: Redacted for privacy DT. James D. White Part I A total synthesis of the insect antifeedant(±)-cinnamodial ( ) and of the related drimanesesquiterpenes (±)-isodrimenin (67) and (±)-fragrolide (72)are described from the diene diester 49. Hydro- boration of 49 provided the C-6oxygenation and the trans ring junction in the form of alcohol 61. To confirm the stereoselectivity of the hydroboration, 61 was convertedto both (t)-isodrimenin (67) and (±)-fragrolide (72) in 3 steps. A diisobutylaluminum hydride reduction of 61 followed by a pyridiniumchlorochromate oxidation and treatment with lead tetraacetate yielded the dihydrodiacetoxyfuran102. The base induced elimination of acetic acid preceded theepoxidation and provided 106 which contains the desired hydroxy dialdehydefunctionality of cinnamodial in a protected form. The epoxide 106 was opened with methanol to yield the acetal 112. Reduction, hydrolysis and acetylation of 112 yielded (t)- cinnamodial in 19% overall yield. Part II - Various N- acyl- 2- thionothiazolidineswere prepared and photo- lysed in the presence of ethanol to provide the corresponding ethyl esters. The photochemical activation of the carboxyl function via these derivatives appears, for practical purposes, to be restricted tocases where a-keto hydrogen abstraction and subsequent ketene formation is favored by acyl substitution. Part 1 The Total Synthesis of (±)-Cinnamodial and Related Drimane Sesquiterpenes. Part 2 Photochemical Activation of the Carboxyl Group via N-Acy1-2-thionothiazolidines. -

Epoxidation of Olefins Using Molecular Oxygen
Epoxidation of Olefins Using Molecular Oxygen Zhongxing Huang Sep 16th, 2015 1 Major Challenge in Catalysis Key points . Low temperature . Selective May 31st, 1993, C&EN News. 2 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product Iron-Containing Enzymes, RSC Publishing, 2011. 3 Most Ancient Chemistry-Oxygenase Dioxygenase incorporates both oxygen atoms into the product Shen, B.; Gould, S. J. Biochemistry 1991, 30, 8936. Gould, S. J.; Kirchmeier, M. J.; LaFever, R. E. J. Am. Chem. Soc. 1996, 118, 7663. 4 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product 5 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product Ideal Dioxygenase incorporates both oxygen atoms into the epoxide 6 Most Ancient Chemistry-Oxygenase Monooxygenase catalyzes the incorporation of one atom of oxygen into the product . Ideal system should avoid use of reductants Dioxygenase incorporates both oxygen atoms into the product Ideal Dioxygenase incorporates both oxygen atoms into the epoxide 7 Industrial Process- EO and PO . Top chemicals produced in US (2004,103 ton) 1 sulfuric acid 35954 2 nitrogen 30543 3 ethylene 25682 4 oxygen 25568 5 propylene 15345 6 chlorine 12166 7 ethylene dichloride 12163 8 phosphoric acid 11463 9 ammonia 10762 10 sodium hydroxide 9508 11 benzene 7675 12 nitric acid 6703 13 ammonium nitrate 6021 14 ethylbenzene 5779 15 urea 5755 16 styrene 5394 17 hydrochloric acid 5012 18 ethylene oxide 3772 19 cumene 3736 20 ammonium sulfate 2643 8 Industrial Process- EO and PO . -

20 More About Oxidation–Reduction Reactions
More About 20 Oxidation–Reduction Reactions OOC n important group of organic reactions consists of those that O A involve the transfer of electrons C from one molecule to another. Organic chemists H OH use these reactions—called oxidation–reduction reactions or redox reactions—to synthesize a large O variety of compounds. Redox reactions are also important C in biological systems because many of these reactions produce HH energy. You have seen a number of oxidation and reduction reactions in other chapters, but discussing them as a group will give you the opportunity to CH3OH compare them. In an oxidation–reduction reaction, one compound loses electrons and one com- pound gains electrons. The compound that loses electrons is oxidized, and the one that gains electrons is reduced. One way to remember the difference between oxidation and reduction is with the phrase “LEO the lion says GER”: Loss of Electrons is Oxi- dation; Gain of Electrons is Reduction. The following is an example of an oxidation–reduction reaction involving inorganic reagents: Cu+ + Fe3+ ¡ Cu2+ + Fe2+ In this reaction,Cu+ loses an electron, so Cu+ is oxidized. Fe3+ gains an electron, so Fe3+ is reduced. The reaction demonstrates two important points about oxidation– reduction reactions. First, oxidation is always coupled with reduction. In other words, a compound cannot gain electrons (be reduced) unless another compound in the reaction simultaneously loses electrons (is oxidized). Second, the compound that is oxidized (Cu+) is called the reducing agent because it loses the electrons that are used to reduce the other compound (Fe3+). Similarly, the compound that is reduced (Fe3+) is called the oxidizing agent because it gains the electrons given up by the other compound (Cu+) when it is oxidized. -

Reactions of Epoxides with Neighbouring Nucleophiles
REACTIONS OF EPOXIDES WITH NEIGHBOURING NUCLEOPHILES A thesis presented for the degree of Doctor o.f Philosophy in Chemistry in the University of Canterbury, Christchurch, New Zealand. by W.H. Swallow 1972 CONTENTS ABSTRACT INTRODUCTION 1 Acid catalysed reactions of epoxides 1 (i) Mechanism of acid catalysed reactions of epoxides with nucleophiles 4 (ii) Ring formation by acid catalysed intramolecular nucleophilic attack of epoxides 10 (iii) Mechanism of acid catalysed rearrangements of epoxides to give carbonyl compounds. 14 Objectives and work undertaken 26 NEIGHBOURING HYDROXYL GROUPS 28 Preparation of the epoxyalcohols 28 The acid catalysed reactions of the epoxyalcohols 36 Rearrangements of epoxyheptanols (26,32) and epoxy- hexanols (24,30) 37 Mechanism of rearrangements of epoxyheptanols (26,32} and epoxyhexanols (24,30) 40 Rearrangements of epoxypentanols (22,28} 42 Mechanism of rearrangements of epoxypentanols (22,28) 44 THE ACID CATALYSED REARRANGEMENTS OF EPOXIDES WITH NEIGHBOURING ACETATE GROUPS 53 Introduction 53 Mechanisms of the acid catalysed reactions of epoxides with neighbouring ester groups 54 The acid catalysed reactions of epoxyacetates 59 Rearrangements of epoxypentanol acetates {23,29) 60 Rearrangements of epoxyhexanol acetates (25,31} 61 Rearrangements of epoxyheptanol acetates (27,33) 64 18 Rearrangements of o enriched epoxyacetates 65 Mechanism of formation of tetrahydrofuranol derivatives from epoxyacetates 66 Mechanism of formation of fluorohydrins and carbonyl compounds from epoxyacetates 73 EXPERIMENTAL 76 REFERENCES 109 ABSTRACT The syntheses and acid catalysed rearrangements of cis- and trans-3,4-epoxypentan-1-ols (28,22), 4,5-epoxyhexan-1-ols (30,24) and 5,6-epoxyheptan-1-ols (32,26) have been described. Isomerizations of these compounds gave cyclic ether derivatives as the major products. -

Synthesis and Polymerization of Several Ester Substituted Epoxides. John, Muggee University of Massachusetts Amherst
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-1982 Synthesis and polymerization of several ester substituted epoxides. John, Muggee University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Muggee, John,, "Synthesis and polymerization of several ester substituted epoxides." (1982). Doctoral Dissertations 1896 - February 2014. 670. https://scholarworks.umass.edu/dissertations_1/670 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. SYNTHESIS AND POLYMERIZATION OF SEVERAL ESTER SUBSTITUTED EPOXIDES A Dissertation Presented By JOHN MUGGEE Submitted to the Graduate School of the University of Massachusetts in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Pebtuary 1982. Polymer Science and Engineering SYNTHESIS AND POLYMERIZATION OF SEVERAL ESTER SUBSTITUTED EPOXIDES A Dissertation Presented By JOHN MUGGEE Approved as to style and content by: Otto Vogl,/ Chairpd^rson of Committee R. S. Stein, Member Polymer Science and Engineering 11( JOHN MUGGEE All rights reserved . ACKNOWLEDGMENTS The author would like to offer his deepest thanks and gratitude to his parents for their love and guidance thoughout his life, and to Professor Otto Vogl for his friendship and professional guidance during the course of this research. The efforts and helpful suggestions con- tributed by Professors Richard S. Stein and James C. W. Chien are also appreciated. The author would also like to thank his laboratory coworkers for making this an interest ing and productive period of his life. -

United States Patent Office 2,589,245 Amide-Epoxide Compositions, Etc
Patented Mar. 18, 1952 2,589,245 UNITED STATES PATENT OFFICE 2,589,245 AMIDE-EPOXIDE COMPOSITIONS, ETC. Sylvan Owen Greenlee, Louisville, Ky., assignor to Devoe & Raynolds Company, Inc., Louisville, Ky., a corporation of New York No Drawing. Application December 3, 1945, Serial No. 632,595 Claims. (Cl. 260-47) 1. 2 This invention relates to new complex amide Another object of the invention is the produc epoxide compositions, and more particularly to tion of Compositions and reaction products of Such compositions capable of conversion into in complex epoxides with amides and amino-amides Soluble, infusible products, and valuable for use of higher unsaturated fatty acids. in making varnishes and protective coatings, in Another object of the invention is the produc making molding compositions and articles, as ad tion of Solutions of such amide-epoxy composi hesives, and in making films and fibres, etc. The tions for use in making warnishes and protective invention includes various new amide-epoxy com coatings, impregnating solutions, films, filaments, positions and reaction products and articles and etc. products made therefrom. 10 Another object of the invention is the produc The new amide-epoxy compositions and prod tion of molding mixtures and compositions ucts are made by reacting amides with complex capable of conversion into infusible, molded ar epoxides produced by the reaction of polyhydric ticles and products, and the articles and prod phenols with polyfunctional halohydrins or with lucts So produced. polyepoxides to form complex reaction products 5 Other objects of the invention and the nature containing terminal epoxide groups. Such epox and advantages of the invention will further ap ide products with which the amides are reacted pear from the following more detailed descrip are advantageously complex polymeric products tion. -

Reactions of Alkenes
Reactions of Alkenes Alkenes generally react in an addition mechanism (addition – two new species add to a molecule and none leave) R X Y X Y H R R R H Have already observed using a H+ electrophile (HBr or H+/H2O) that a carbocation intermediate is generated which directs the regiochemistry Whenever a free carbocation intermediate is generated there will not be a stereopreference due to the nucleophile being able to react on either lobe of the carbocation (already observed this with SN1 and E1 reactions) Br Br H+ H H3C Br CH2CH3 Obtain racemic mixture of this regioisomer Reactions of Alkenes There are three questions to ask for any addition reaction R X Y X Y H R R R H 1) What is being added? (what is the electrophile?) 2) What is the regiochemistry? (do the reagents add with the X group to the left or right?) 3) What is the stereochemistry? (do both the X and Y groups add to the same side of the double bond or opposite sides?) All of these questions can be answered if the intermediate structure is known Reactions of Alkenes Dihalogen compounds can also react as electrophiles in reactions with alkenes Possible partial bond structures + !+ Br Br ! Br Br Br !+ or Br !+ More stable partial positive charge Experimentally it is known, however, that rearrangements do nor occur with Br2 addition -therefore free carbocations must not be present The large size and polarizability of the halogen can stabilize the unstable carbocation With an unsymmetrical alkene, however, both bonds to the bromine need not be equivalent Called a “Bromonium” ion -

Synthesis and Reactions = R-Br in Our Course)
Chem 201/Beauchamp Reactions and Mechanisms Worksheet 1 Starting organic compounds Br Br Br Br CH4 1o given 1o given o 1 given rearrangement example R-X Compounds (synthesis and reactions = R-Br in our course) 1. Make other R-Br compounds (nine examples for us, 3 primary examples given above and six more made below) a. R-Br from alkanes using free radical substitution (three part sequence: 1. Initiation 2. Propagation (2 steps) 3. termination 1. initiation - high energy photon ruptures the weakest bond (= halogen bond = homolytic cleavage) atoms bond energy Br-Br 46 3 Br h sp H3C-H 105 Br Br Br sp3 1o C-H 98 sp3 2o C-H 95 sp3 3o C-H 92 2a. propagation (step 1) - bromine atom abstracts H from weakest C-H bond and makes an H-Br bond sp3 C-Br 68 H-Br 88 H2 CH2 C Br H Br H3C H3C H 2b. propagation (step 2) - carbon free radical abstracts Br atom from bromine molecule and makes a C-Br bond CH Br H2 2 C H3C Br H3C Br Br 3. termination - two reactive free radicals find one another and combine H2 C CH CH2 H C 3 2 H C C H3C 3 CH3 H2 H2 C CH2 Br H C Br H3C 3 Other R-Br to make from our starting alkanes. Six possible RBr from our starting alkanes plus three primary RBr are given = 9 total. CH3 Br CH3 H CH3 H C Br 2 3 C C H C H C CH 3 H3C Br CH 3 H3C Br H C Br C Br 3 H2 Br h Br2 h Br 2 Br2 h Br 2 h 2 h Br2 h CH4 shown above There is no easy way to make a primary R-Br compound in our course yet (from our given starting structures). -

A Short Note on Epoxide
ry: C ist urr m en e t h R C e c s i e n a a r c g r h O ISSN: 2161-0401 Organic Chemistry: Current Research Short Communication A Short Note on Epoxide Sudha M Department of Organic Chemistry, Vivekavardhini College, Kothagudem, Telangana, India ABSTRACT Epoxide, cyclic ether with a three-membered ring. the essential structure of an epoxide contains an oxygen atom attached to two adjacent carbon atoms of a hydrocarbon. The strain of the three-membered ring makes an epoxide far more reactive than a typical acyclic ether. Ethylene oxide is economically the foremost important epoxide and is formed from oxidation of ethylene over a silver catalyst. it's used as a fumigant and to form antifreeze, glycol , and other useful compounds. Keywords: Uses; Chlorohydrin INTRODUCTION the corresponding cis-alkene. This hydration of an epoxide doesn't change the oxidation number of any atoms or Another important industrial route to epoxides requires a two- groups. step process. First, an alkene is converted to a, and second, the chlorohydrin is treated with a base to eliminate acid , giving the Epoxides also can be opened by other anhydrous acids (HX) to epoxide; this is often the tactic wont to make propylene oxide. make a trans halohydrin. When both the epoxide carbons are either primary or secondary the halogen anion will attack the Epoxides are easily opened, under acidic or basic conditions, to less substituted carbon and an SN2 like reaction. However, offer a spread of products with useful functional groups. for if one among the epoxide carbons is tertiary, the halogen anion instance , the acid- or base-catalyzed hydrolysis of propylene will primarily attack the tertialy cabon during a SN1 like oxide gives propanediol .