Role and Modulation of NK Cells in Multiple Myeloma
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

HLA Mismatching Favoring Host-Versus-Graft NK Cell Activity Via KIR3DL1 Is Associated With
HLA mismatching favoring host-versus-graft NK cell activity via KIR3DL1 is associated with improved outcomes following lung transplantation John R. Greenland1,2,6, Haibo Sun3, Daniel Calabrese2, Tiffany Chong2, Jonathan P. Singer2, Jasleen Kukreja4, Steven R. Hays2, Jeffrey A. Golden2,4, George H. Caughey1,2,5, Jeffrey M. Venstrom2, Raja Rajalinginam3 1 Medical Service, Veterans Affairs Medical Center, San Francisco CA, 94121 2 Department of Medicine, University of California, San Francisco CA, 94143 3 Immunogenetics and Transplantation Laboratory, Department of Surgery, University of California, San Francisco CA, 94143 4 Department of Surgery, University of California, San Francisco CA, 94143 5 Cardiovascular Research Institute, University of California, San Francisco CA, 94143 6 Corresponding author: [email protected] Running Title: HVG NK cell activity in lung transplantation Abbreviations: antigen presenting cell (APC), bronchiolitis obliterans syndrome (BOS), bronchoalveolar lavage (BAL), chronic lung allograft dysfunction (CLAD), confidence interval (CI), forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), hazard ratio (HR), host-versus-graft (HVG), Killer cell immunoglobulin-like receptor (KIR), Major Histocompatibility Complex (MHC), Natural Killer (NK), restrictive allograft syndrome (RAS), United Network for Organ Sharing (UNOS), University of California, San Francisco (UCSF) Abstract Chronic lung allograft dysfunction (CLAD) is linked to rejection and limits survival following lung transplantation. HLA-Bw4 recipients of HLA-Bw6 grafts have enhanced host-versus-graft (HVG) NK cell activity mediated by KIR3DL1 ligand. Because natural killer (NK) cells may promote tolerance by depleting antigen-presenting cells, we hypothesized improved outcomes for HLA-Bw4 recipients of HLA- Bw6 grafts. We evaluated differences in acute cellular rejection (ACR) and CLAD-free survival across 252 KIR3DL1+ recipients from UCSF. -

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -
![Anti-NCR1 Antibody [29A1.4] (FITC) (ARG23568)](https://docslib.b-cdn.net/cover/3482/anti-ncr1-antibody-29a1-4-fitc-arg23568-113482.webp)
Anti-NCR1 Antibody [29A1.4] (FITC) (ARG23568)
Product datasheet [email protected] ARG23568 Package: 50 μg anti-NCR1 antibody [29A1.4] (FITC) Store at: 4°C Summary Product Description FITC-conjugated Rat Monoclonal antibody [29A1.4] recognizes NCR1. Rat anti Mouse CD335 antibody, clone 29A1.4 recognizes natural killer cell p46-related protein (NKp46), otherwise known as CD335, which is uniquely expressed by resting and activated natural killer (NK) cells, but no expression of CD335 has been detected on B cells, T cells, monocytes or granulocytes. It is a major NK cell lysis receptor for autologous pathogen-infected and tumor target cells during natural cytotoxicity responses. Ligands of CD335 include viral hemagglutinins (HAs) and heparan sulfate proteoglycans (HSPGs) on the surface of tumor cells. Staining with Rat anti mouse CD335 (29A1.4) is not strain specific and the antibody has been used to stain C57BL/6, SJL, CBA/CA and BALB/c strains. Clone 29A1.4 also activates NK cells in vitro. It does not deplete NK cells in vivo. Tested Reactivity Ms Tested Application FACS Host Rat Clonality Monoclonal Clone 29A1.4 Isotype IgG2a Target Name NCR1 Antigen Species Mouse Immunogen NKP46-IgG1 Fc fusion protein. Conjugation FITC Alternate Names CD antigen CD335; Natural killer cell p46-related protein; Lymphocyte antigen 94 homolog; Natural cytotoxicity triggering receptor 1; hNKp46; NKP46; NKp46; NK cell-activating receptor; LY94; CD335; NK- p46 Application Instructions Application table Application Dilution FACS 1:2 - 1:5 Application Note FACS: Use 10 µl of the suggested working dilution to label 10^6 cells in 100 µl. * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. -

One-Year Follow-Up of Natural Killer Cell Activity in Multiple Myeloma
One-Year Follow-Up of Natural Killer Cell Activity in Multiple Myeloma Patients Treated With Adjuvant Lenalidomide Therapy Laurie Besson, Emily Charrier, Lionel Karlin, Omran Allatif, Antoine Marçais, Paul Rouzaire, Lucie Belmont, Michel Attal, Christine Lombard, Gilles Salles, et al. To cite this version: Laurie Besson, Emily Charrier, Lionel Karlin, Omran Allatif, Antoine Marçais, et al.. One- Year Follow-Up of Natural Killer Cell Activity in Multiple Myeloma Patients Treated With Adjuvant Lenalidomide Therapy. Frontiers in Immunology, Frontiers, 2018, 9, pp.704. 10.3389/fimmu.2018.00704. hal-01833354 HAL Id: hal-01833354 https://hal.archives-ouvertes.fr/hal-01833354 Submitted on 22 Oct 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License ORIGINAL RESEARCH published: 13 April 2018 doi: 10.3389/fimmu.2018.00704 One-Year Follow-Up of Natural Killer Cell Activity in Multiple Myeloma Patients Treated With Adjuvant Lenalidomide Therapy Laurie Besson1,2,3,4,5,6†, Emily Charrier1,2,3,4,5,6†, -

Expression Tissue
The Journal of Immunology Killer Cell Ig-Like Receptor and Leukocyte Ig-Like Receptor Transgenic Mice Exhibit Tissue- and Cell-Specific Transgene Expression1 Danny Belkin,* Michaela Torkar,* Chiwen Chang,* Roland Barten,* Mauro Tolaini,† Anja Haude,* Rachel Allen,* Michael J. Wilson,* Dimitris Kioussis,† and John Trowsdale2* To generate an experimental model for exploring the function, expression pattern, and developmental regulation of human Ig-like activating and inhibitory receptors, we have generated transgenic mice using two human genomic clones: 52N12 (a 150-Kb clone encompassing the leukocyte Ig-like receptor (LILR)B1 (ILT2), LILRB4 (ILT3), and LILRA1 (LIR6) genes) and 1060P11 (a 160-Kb clone that contains ten killer cell Ig-like receptor (KIR) genes). Both the KIR and LILR families are encoded within the leukocyte receptor complex, and are involved in immune modulation. We have also produced a novel mAb to LILRA1 to facilitate expression studies. The LILR transgenes were expressed in a similar, but not identical, pattern to that observed in humans: LILRB1 was expressed in B cells, most NK cells, and a small number of T cells; LILRB4 was expressed in a B cell subset; and LILRA1 was found on a ring of cells surrounding B cell areas on spleen sections, consistent with other data showing monocyte/macrophage expression. KIR transgenic mice showed KIR2DL2 expression on a subset of NK cells and T cells, similar to the pattern seen in humans, and expression of KIR2DL4, KIR3DS1, and KIR2DL5 by splenic NK cells. These observations indicate that linked regulatory elements within the genomic clones are sufficient to allow appropriate expression of KIRs in mice, and illustrate that the presence of the natural ligands for these receptors, in the form of human MHC class I proteins, is not necessary for the expression of the KIRs observed in these mice. -

Role for NK Cells Inflammatory Response Reveals a Critical A
A Fatal Cytokine-Induced Systemic Inflammatory Response Reveals a Critical Role for NK Cells This information is current as William E. Carson, Haixin Yu, Julie Dierksheide, Klaus of September 28, 2021. Pfeffer, Page Bouchard, Reed Clark, Joan Durbin, Albert S. Baldwin, Jacques Peschon, Philip R. Johnson, George Ku, Heinz Baumann and Michael A. Caligiuri J Immunol 1999; 162:4943-4951; ; http://www.jimmunol.org/content/162/8/4943 Downloaded from References This article cites 61 articles, 37 of which you can access for free at: http://www.jimmunol.org/content/162/8/4943.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1999 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. A Fatal Cytokine-Induced Systemic Inflammatory Response Reveals a Critical Role for NK Cells1 William E. Carson,2*† Haixin Yu,† Julie Dierksheide,‡ Klaus Pfeffer,§ Page Bouchard,¶ Reed Clark,| Joan Durbin,| Albert S. -

Receptor Nkp46 Cells by the NK Β Murine Pancreatic Recognition And
Recognition and Killing of Human and Murine Pancreatic β Cells by the NK Receptor NKp46 This information is current as Chamutal Gur, Jonatan Enk, Sameer A. Kassem, Yaron of September 27, 2021. Suissa, Judith Magenheim, Miri Stolovich-Rain, Tomer Nir, Hagit Achdout, Benjamin Glaser, James Shapiro, Yaakov Naparstek, Angel Porgador, Yuval Dor and Ofer Mandelboim J Immunol 2011; 187:3096-3103; Prepublished online 17 Downloaded from August 2011; doi: 10.4049/jimmunol.1101269 http://www.jimmunol.org/content/187/6/3096 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2011/08/18/jimmunol.110126 Material 9.DC1 References This article cites 29 articles, 9 of which you can access for free at: http://www.jimmunol.org/content/187/6/3096.full#ref-list-1 Why The JI? Submit online. by guest on September 27, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Recognition and Killing of Human and Murine Pancreatic b Cells by the NK Receptor NKp46 Chamutal Gur,*,†,1 Jonatan Enk,*,1 Sameer A. -

High Mtor Activity Is a Hallmark of Reactive Natural Killer Cells And
RESEARCH ARTICLE High mTOR activity is a hallmark of reactive natural killer cells and amplifies early signaling through activating receptors Antoine Marc¸ais1,2,3,4,5*, Marie Marotel1,2,3,4,5, Sophie Degouve1,2,3,4,5, Alice Koenig1,2,3,4,5, Se´ bastien Fauteux-Daniel1,2,3,4,5, Annabelle Drouillard1,2,3,4,5, Heinrich Schlums6, Se´ bastien Viel1,2,3,4,5,7, Laurie Besson1,2,3,4,5, Omran Allatif1,2,3,4,5, Mathieu Ble´ ry8, Eric Vivier9,10, Yenan Bryceson6,11, Olivier Thaunat1,2,3,4,5, Thierry Walzer1,2,3,4,5* 1CIRI, Centre International de Recherche en Infectiologie - International Center for Infectiology Research, Lyon, France; 2Inserm, U1111, Lyon, France; 3Ecole Normale Supe´rieure de Lyon, Lyon, France; 4Universite´ Lyon 1, Lyon, France; 5CNRS, UMR5308, Lyon, France; 6Centre for Hematology and Regenerative Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, Stockholm, Sweden; 7Laboratoire d’Immunologie, Hospices Civils de Lyon, Centre Hospitalier Lyon Sud, Lyon, France; 8Innate-Pharma, Marseille, France; 9Aix-Marseille Universite´, CNRS, INSERM, CIML, Marseille, France; 10APHM, Hoˆpital de la Timone, Service d’Immunologie, Marseille, France; 11Broegelmann Research Laboratory, The Gades Institute, University of Bergen, Bergen, Norway Abstract NK cell education is the process through which chronic engagement of inhibitory NK *For correspondence: cell receptors by self MHC-I molecules preserves cellular responsiveness. The molecular [email protected] (AM); mechanisms responsible for NK cell education remain unclear. Here, we show that mouse NK cell [email protected] (TW) education is associated with a higher basal activity of the mTOR/Akt pathway, commensurate to Competing interest: See the number of educating receptors. -
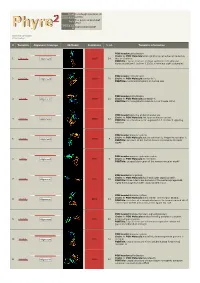
Phyre 2 Results for A1YIY0
Email [email protected] Description A1YIY0 Tue Jul 30 13:19:15 BST Date 2013 Unique Job 1035bc4b501530df ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:cell adhesion Chain: A: PDB Molecule:down syndrome cell adhesion molecule 1 c3dmkA_ Alignment 100.0 14 (dscam) isoform PDBTitle: crystal structure of down syndrome cell adhesion molecule (dscam)2 isoform 1.30.30, n-terminal eight ig domains PDB header:cell adhesion 2 c2om5A_ Alignment 100.0 20 Chain: A: PDB Molecule:contactin 2; PDBTitle: n-terminal fragment of human tax1 PDB header:cell adhesion 3 c3jxaA_ Alignment 100.0 21 Chain: A: PDB Molecule:contactin 4; PDBTitle: immunoglobulin domains 1-4 of mouse cntn4 PDB header:signaling protein/transferase Chain: A: PDB Molecule:tek tyrosine kinase variant; 4 c4k0vA_ 100.0 12 Alignment PDBTitle: structural basis for angiopoietin-1 mediated signaling initiation PDB header:immune system Chain: A: PDB Molecule:natural cytotoxicity triggering receptor 1; 5 c1p6fA_ 100.0 9 Alignment PDBTitle: structure of the human natural cytotoxicity receptor nkp46 PDB header:immune system/receptor 6 c1ollA_ Alignment 99.9 9 Chain: A: PDB Molecule:nk receptor; PDBTitle: extracellular region of the human receptor nkp46 PDB header:viral protein Chain: A: PDB Molecule:hoc head outer capsid protein; 7 c3shsA_ 99.9 18 Alignment PDBTitle: three n-terminal domains of the bacteriophage rb49 highly immunogenic2 outer capsid protein (hoc) PDB header:immune system Chain: E: PDB Molecule:natural -

Receptor Nkp46/NCR1 Tumors in the Absence of the NK-Activating
The Journal of Immunology Enhanced In Vivo Growth of Lymphoma Tumors in the Absence of the NK-Activating Receptor NKp46/NCR11 Gili G. Halfteck, Moran Elboim, Chamutal Gur, Hagit Achdout, Hormas Ghadially, and Ofer Mandelboim2 The in vitro elimination of virus-infected and tumor cells by NK cells is regulated by a balance between signals conveyed via specific inhibitory and activating receptors. Whether NK cells and specifically the NK-activating receptor NKp46 (NCR1 in mice) are directly involved in tumor eradication in vivo is still largely unknown. Since the NKp46/NCR1 tumor ligands have not been identified yet, we use a screening technique to identify functional ligands for NKp46/NCR1 which is based on a cell reporter assay and discover a NCR1 ligand in the PD1.6 lymphoma line. To study whether NKp46/NCR1 is important for the eradication of PD1.6 lymphoma in vivo, we used the Ncr1 knockout Ncr1gfp/gfp mice generated by our group. Strikingly, all Ncr1 knockout mice developed growing PD1.6 tumors, whereas initial tumor growth was observed in the wild-type mice and tumors were completely rejected as time progressed. The growth of other lymphoma cell lines such as B10 and EL4 was equivalent between the Ncr1 knockout and wild-type mice. Finally, we show that PD1.6 lymphoma cells are less killed both in vitro and in vivo in the absence of NKp46/NCR1. Our results therefore reveal a crucial role for NKp46/NCR1 in the in vivo eradication of some lymphoma cells. The Journal of Immunology, 2009, 182: 2221–2230. t was hypothesized that the immune system surveys the body reduced resistance to transplanted tumor cell lines (20–22). -

Platelets Impair Natural Killer Cell Reactivity and Function in Endometriosis Through Multiple Mechanisms
Human Reproduction, Vol.32, No.4 pp. 794–810, 2017 Advanced Access publication on February 9, 2017 doi:10.1093/humrep/dex014 ORIGINAL ARTICLE Gynaecology Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms Yanbo Du, Xishi Liu, and Sun-Wei Guo* Shanghai Obstetrics and Gynecology Hospital, Fudan University Shanghai College of Medicine, 419 Fangxie Road, Shanghai 200011, China *Correspondence Address. Shanghai Obstetrics and Gynecology Hospital, Fudan University Shanghai College of Medicine, Shanghai 200011, China. Fax: 86-21-6345-5090; E-mail: [email protected] Submitted on October 28, 2016; resubmitted on December 27, 2016; accepted on January 13, 2017 STUDY QUESTION: Do platelets have any role in the reduced cytotoxicity of natural killer (NK) cells in endometriosis? SUMMARY ANSWER: Platelets impair NK cell reactivity and function in endometriosis through multiple mechanisms. WHAT IS KNOWN ALREADY: Platelets play an important role in the development of endometriosis, and platelet-derived transforming growth factor-β1 (TGF-β1) suppresses the expression of NK Group 2, Member D (NKG2D) on NK cells, resulting in reduced cytotoxicity in women with endometriosis. STUDY DESIGN SIZE, DURATION: Experiments on mice with induced endometriosis in which either platelets, NK cells or both were depleted and controls (none depleted). In vitro experiments with NK cells, platelets and, as target cells, endometriotic epithelial cell and endo- metrial stromal cell lines. PARTICIPANTS/MATERIALS SETTING METHODS: Immunohistochemistry analysis of ectopic endometrial tissues from mice with induced endometriosis receiving either platelet depletion (PD), NK cell depletion, or both or none. Immunofluorescence, flow cytometry and gene expression analysis for major histocompatibility complex class I (MHC-I) expression in target cells. -

Deciphering the Transcriptional Switches of Innate Lymphoid Cell Programming: the Right Factors at the Right Time
Genes and Immunity (2015) 16, 177–186 © 2015 Macmillan Publishers Limited All rights reserved 1466-4879/15 www.nature.com/gene REVIEW Deciphering the transcriptional switches of innate lymphoid cell programming: the right factors at the right time AWY Lim and ANJ McKenzie Innate lymphoid cells (ILCs) are increasingly recognised as an innate immune counterpart of adaptive T-helper (TH) cells. In addition to their similar effector cytokine production, there is a strong parallel between the transcription factors that control the differentiation of TH1, TH2 and TH17 cells and ILC groups 1, 2 and 3, respectively. Here, we review the transcriptional circuit that specifies the development of a common ILC progenitor and its subsequent programming into distinct ILC groups. Notch, GATA-3 (GATA-binding protein 3), Nfil3 (nuclear factor interleukin-3) and Id2 (inhibitor of DNA-binding 2) are identified as early factors that suppress B- and T-cell potentials and are turned on in favour of ILC commitment. Natural killer cells, which are the cytotoxic ILCs, develop along a pathway distinct from the rest of the helper-like ILCs that are derived from a common progenitor to all helper-like ILCs (CHILPs). PLZF− (promyelocytic leukaemia zinc-finger) CHILPs give rise to lymphoid tissue inducer cells, while PLZF+ CHILPs have multilineage potential and could give rise to ILCs 1, 2 and 3. Such lineage specificity is dictated by the controlled expression of T-bet (T-box expressed in T cells), RORα (retinoic acid receptor-related orphan nuclear receptor-α), RORγt (retinoic acid receptor- related orphan nuclear receptor-γt) and AHR (aryl hydrocarbon receptor).