Isuprel™ Rx Only Isoproterenol Hydrochloride Injection, USP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Murine Model of Experimental Metastasis to Bone and Bone Marrow1
(CANCER RESEARCH 48. 6876-6881, December 1, 1988] A Murine Model of Experimental Metastasis to Bone and Bone Marrow1 Francisco Arguello,2 Raymond B. Baggs, and Christopher N. Frantz3 Department of Pediatrics and the Cancer Center ¡F.A., C. N. F.], and Department of Pathology and Division of Laboratory Animal Medicine [R. B. B.], University of Rochester School of Medicine and Dentistry, Rochester, New York 14642 ABSTRACT In this model, tumor cells are injected in the mouse tail vein. Most of the cells die, but a few survive, grow, and form tumor Bone is a common site of metastasis in human cancer. A major colonies in the lung. The colonies are counted to quantify the impediment to understanding the pathogenesis of bone metastasis has experimental metastasis. The ability of the lung to kill the vast been the lack of an appropriate animal model. In this paper, we describe an animal model in which B16 melanoma cells injected in the left cardiac majority of injected tumor cells (11, 12) may preclude the use ventricle reproducibly colonize specific sites of the skeletal system of of i.v. injection to study metastasis to extrapulmonary organs. mice. Injection of 10*cells resulted in melanotic tumor colonies in most Bone metastasis models have involved direct injection of tumor organs, including the skeletal system. Injection of IO4 or fewer cells cells into the medullary cavity of bones (13, 14) or into the rat resulted in experimental metastasis almost entirely restricted to the abdominal aorta (15). In this paper, we describe our experience skeletal system and ovary. -

I SHORT TERM ELECTRICAL STIMULATION for ISOGRAFT PERIPHERAL NERVE REPAIR and FUNCTIONAL RECOVERY a Thesis Presented to the Gradu
SHORT TERM ELECTRICAL STIMULATION FOR ISOGRAFT PERIPHERAL NERVE REPAIR AND FUNCTIONAL RECOVERY A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment Of the Requirements for the Degree Master of Science in Engineering, Biomedical Concentration Galina Y. Pylypiv May, 2018 i SHORT TERM ELECTRICAL STIMULATION FOR ISOGRAFT PERIPHERAL NERVE REPAIR AND FUNCTIONAL RECOVERY Galina Y. Pylypiv Thesis Approved: Accepted: Advisor Dean of the College of Engineering Dr. Rebecca Kuntz Willits Dr. Donald Visco Committee Member Executive Dean of the Graduate School Dr. Matthew Becker Dr. Chand Midha Committee Member Date Dr. Ge Zhang Biomedical Engineering Department Chair Dr. Brian Davis ii ABSTRACT Electrical stimulation (ES) has previously demonstrated promising effects on peripheral nerve repair through enhanced neurite growth in vitro and shortened recovery time in vivo. In this study, we aimed to evaluate the effect of intraoperative short term ES on a clinically relevant isograft-repair model of a rodent peripheral nerve. In our model, an isograft was used to repair a 13 mm sciatic nerve gap-defect in adult male rats. Intraoperative ES was applied for 10 min at 24 V/m-DC to the experimental group and no stimulation was applied to the control group. We evaluated biweekly functional recovery over 12 weeks for motor function, using the sciatic functional index and external postural thrust. Sensory function was evaluated using a thermal stimulus. Motor nerves are more heavily myelinated and regenerate more quickly, while sensory nerves are less myelinated and have a slower recovery time. Structural repair outcomes were evaluated through histological examination of the sciatic nerves and gastrocnemius muscles at 6 and 12-week time points. -

Routine Dosing in Rodents
Routine Dosing in Rodents University of South Florida February 2008 OBJECTIVES • To provide an overview of basic restraint and handling techniques in rodent species. • To demonstrate common routes of injection and oral dosing procedures in rodent species. Rat Restraint “V” Hold • Held between "v" formed by index and second finger. • Supported by thumb and ring finger under elbows. USES/ADVANTAGE • Intraperitoneal and oral dosing. • Less confining than other types of restraint, e.g., restrainers/scruffing. • Avoids unnecessary stress of surgical incision sites, e.g., thoracic, abdominal, neck regions. Rat Restraint “Scruff” Hold • Held by bunching the skin over the shoulder blades. Uses/Advantages • Works well for subcutaneous injection • Not well tolerated in obese or large animals • Places stress on incision sites of the neck and chest Rat Restrainers • DecapiCone® bag • Plastic Commercial Holder • Towel Arrows indicate breathing holes for each device. These types restrain without enclosing the head. Rat Restrainers (Cont’d) Commercial Plastic (round) Adjustable stoppers “tail in” (small rat) Rat is loaded tail-in or head-in. Rat size, loaded direction, and stopper position combine to allow access to tail vein. “head in” (large rat) Rat Restrainers (Cont’d) Stopper is at half- Can be used with the way point of “stopper” positioned midway for hindquarter restrainer, bleeds or injections. preventing rat from moving The animals head is forward. Rat is placed within the device positioned “head to prevent biting, while in.” leaving access to the hindquarters. RESTRAINERS (cont’d.) Flat-Bottom Hard Plastic Restrainer/Commercial (DecapiCone ®) Bag • Both prevent rat from rolling over while in restraint. • Commercial bag originally developed for humane decapitation. -

Unit 4: Medication Administration Fundamental of Nursing
Unit 4: Medication Administration Fundamental of Nursing Unit 4: Medication Administration: Medication: Is a substance administered for the diagnosis, cure, treatment, relief, or prevention of disease. Six Rights of Medication Administration After paramedics have received the medication or fluid order, they should then administer the drug in question. In performing drug administration, pre-hospital care providers adhere to the six rights of medication administration: 1. Right patient 2. Right medication 3. Right dose 4. Right route 5. Right time 6. Right documentation Basic principle of nurse on drugs administration 1. The nurse must know the drug's prescribed dose, method of administration, actions, expected therapeutic effect, possible interactions with other drugs, and adverse effects. 2. The nurse must know the institution's administration procedures for the client's welfare and the nurse's legal protection. 3. The nurse must Review physician's order for completeness the client's name, date of the order, name of the drug, dose, rout, time of administration, and the physician's signature. 1 Unit 4: Medication Administration Fundamental of Nursing 4. The nurse discusses the medication and its actions with the client; recheck the medication order if the client disagrees with the dose or the physician's order. 5. The nurse must check the physician's order against the client's medication administration record for accuracy. 6. The nurse gives the patient the right to know about the medication he is receiving and the right to refuse it. Routes of Administration A: Enteral Tract Routes The common enteral routes of administration used in general medical practice are as follows: 1. -

Epinephrine Injection, Solution Hospira, Inc. Disclaimer: This Drug Has Not Been Found by FDA to Be Safe and Effective, and This Labeling Has Not Been Approved by FDA
EPINEPHRINE- epinephrine injection, solution Hospira, Inc. Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- EPINEPHRINE Injection, USP 1 mg/10 mL (0.1 mg/mL) Abboject™ Syringe Fliptop Vial Protect solution from light; do not use the Injection if its color is pinkish or darker than slightly yellow or if it contains a precipitate. Rx only DESCRIPTION Epinephrine Injection, USP is a sterile, nonpyrogenic solution administered parenterally by the intravenous or intracardiac (left ventricular chamber) routes, or via endotracheal tube into the bronchial tree. Each milliliter (mL) contains epinephrine 0.1 mg; sodium chloride 8.16 mg; sodium metabisulfite added 0.46 mg; citric acid, anhydrous 2 mg and sodium citrate, dihydrate 0.6 mg added as buffers. May contain additional citric acid and/or sodium citrate for pH adjustment. pH 3.3 (2.2 to 5.0). Epinephrine Injection, USP is oxygen sensitive. The solution contains no bacteriostat or antimicrobial agent and is intended for use only as a single-dose injection. When smaller doses are required the unused portion should be discarded. Epinephrine Injection, USP is a parenteral adrenergic (sympathomimetic) agent and cardiac stimulant. The drug belongs to the group of endogenous compounds known as catecholamines. Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water. Epinephrine, USP is chemically designated 4-[1-hydroxy-2-(methylamino) ethyl]-1, 2 benzenediol, a white, microcrystalline powder. With acids, it forms salts that are freely soluble in water. -

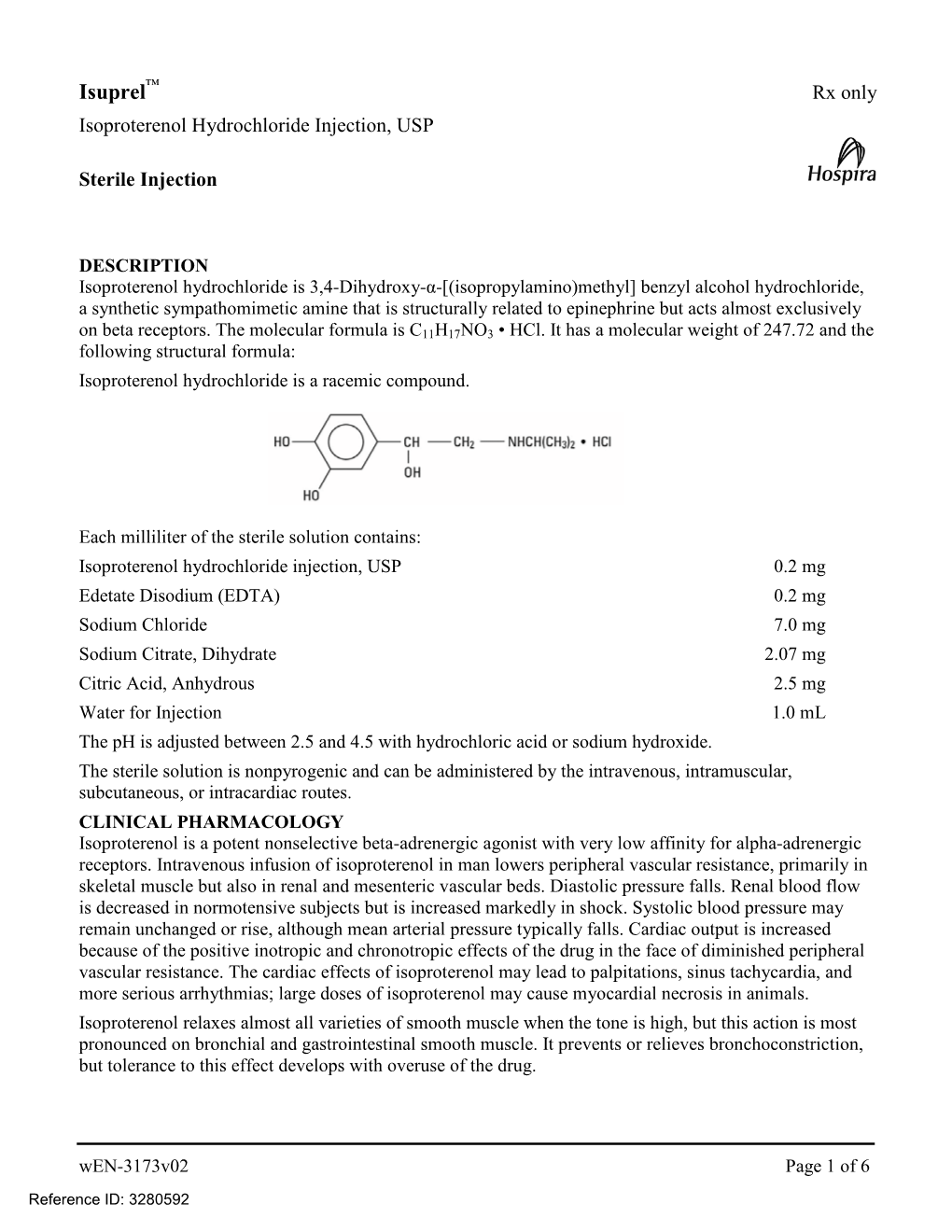
Isoproterenol Hydrochloride Injection, Uspsterile Injectionrx Only
ISOPROTERENOL- isoproterenol hydrochloride injection, solution Cipla USA Inc. ---------- Isoproterenol Hydrochloride Injection, USP Sterile Injection Rx only DESCRIPTION Isoproterenol hydrochloride is 3,4-Dihydroxy-α-[(isopropylamino)methyl] benzyl alcohol hydrochloride, a synthetic sympathomimetic amine that is structurally related to epinephrine but acts almost exclusively on beta receptors. The molecular formula is C11H17NO3 • HCl. It has a molecular weight of 247.72 and the following structural formula: Isoproterenol hydrochloride is a racemic compound. Each milliliter of the sterile solution contains: Isoproterenol hydrochloride USP 0.2 mg Edetate disodium USP (EDTA) 0.2 mg Sodium chloride USP 7.0 mg Sodium citrate, dihydrate USP 2.07 mg Citric acid, anhydrous USP 2.5 mg Hydrochloric acid NF q.s to adjust pH Sodium hydroxide NF q.s to adjust pH Water for Injection USP q.s to 1.0 mL The pH is adjusted between 2.5 and 4.5 with hydrochloric acid NF or sodium hydroxide NF. The sterile solution is nonpyrogenic and can be administered by the intravenous, intramuscular, subcutaneous, or intracardiac routes. CLINICAL PHARMACOLOGY Isoproterenol is a potent nonselective beta-adrenergic agonist with very low affinity for alpha- adrenergic receptors. Intravenous infusion of isoproterenol in man lowers peripheral vascular resistance, primarily in skeletal muscle but also in renal and mesenteric vascular beds. Diastolic pressure falls. Renal blood flow is decreased in normotensive subjects but is increased markedly in shock. Systolic blood pressure may remain unchanged or rise, although mean arterial pressure typically falls. Cardiac output is increased because of the positive inotropic and chronotropic effects of the drug in the face of diminished peripheral vascular resistance. -

Managing Cardiopulmonary Arrest
1 CE Credit Managing Cardiopulmonary Arrest Christopher Norkus, BS, CVT, VTS (ECC), VTS (Anesthesia) ardiopulmonary arrest (CPA) is characterized by abrupt, CPCR Stage 1: Basic Life Support complete failure of the respiratory and circulatory systems. Airway management involves extending the patient’s neck to CThe lack of cardiac output and oxygen delivery to tissues straighten the airway and pulling the patient’s tongue forward. (DO2) can quickly cause unconsciousness and systemic cellular The veterinary staff should quickly examine the upper airway death from oxygen starvation. If left untreated, cerebral hypoxia and initiate suctioning, if necessary. All foreign material or vomit results in complete biologic brain death within 4 to 6 minutes of observed in the patient’s mouth should be cleared immediately. CPA.1,2 Therefore, prompt cardiopulmonary cerebral resuscitation If the patient’s airway is fully obstructed, abdominal thrusts (CPCR) is imperative. Veterinary technicians can play a key role and finger sweeps of the pharynx can help dislodge the obstruction. in ensuring that patients receive this treatment. An emergency tracheotomy, which is performed by a veterinarian, may be necessary if the airway obstruction is not immediately Causes and Clinical Signs resolved. Insertion of a large-gauge needle or intravenous (IV) In dogs and cats, common causes of CPA include anesthetic catheter directly into the trachea below the obstruction, along complications; vagal stimulation; hypovolemia; severe trauma, with oxygen administration, can be useful while the tracheotomy such as pneumothorax; unstable cardiac arrhythmias, such as is being performed. In some cases, material fully obstructing the unstable ventricular tachycardia; severe electrolyte disturbances, airway (e.g., a ball) can be manually removed with long hemostats such as hyperkalemia; cardiorespiratory disorders, such as congestive or Doyen intestinal clamps. -

Data Sheet Template
NEW ZEALAND DATA SHEET 1. PRODUCT NAME IsuprelTM 0.2 mg/mL Solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each mL of the sterile 1:5000 solution contains 0.2 mg of Isoprenaline hydrochloride. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection A colourless, sterile, non pyrogenic solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Isuprel is indicated for: 1. Mild or transient episodes of heart block that do not require electric shock or pacemaker therapy. 2. Serious episodes of heart block and Adams-Stokes attacks (except when caused by ventricular tachycardia of fibrillation). (see section 4.3). 3. Use in cardiac arrest until electric shock or pacemaker therapy, the treatments of choice, are available. (see section 4.3). 4. Bronchospasm occurring during anaesthesia. 5. As an adjunct to fluid and electrolyte replacement therapy and the use of other medicines and procedures in the treatment of hypovolaemic and septic shock, low cardiac output (hypoperfusion) states, congestive heart failure, and cardiogenic shock. (see section 4.4). 4.2 Dose and method of administration Isuprel can be administered by the intravenous, intramuscular, subcutaneous or intracardiac routes. Isuprel should generally be started at the lowest recommended dose and the rate of administration gradually increased if necessary while carefully monitoring the patient. The usual route of administration is by intravenous infusion or bolus intravenous injection. In dire emergencies, the medicine may be administered by intracardiac injection. If time is not of the utmost importance, initial therapy by intramuscular or subcutaneous injection is preferred. Version: 4.0 Page 1 of 11 Elderly patients may be more sensitive to the effects of sympathomimetics and lower doses may be required. -

Epinephrine 1:10,000 0.1 Mg/Ml Injection, USP 10 Ml Prefilled Syringe
EPINEPHRINE- epinephrine injection, solution General Injectables & Vaccines, Inc Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- Epinephrine 1:10,000 0.1 mg/mL Injection, USP 10 mL PreFilled Syringe Description EPINEPHRINE Injection, USP 1 mg/10 mL (0.1 mg/mL) Abboject™ Syringe Fliptop Vial Protect solution from light; do not use the Injection if its color is pinkish or darker than slightly yellow or if it contains a precipitate. Epinephrine Injection, USP is a sterile, nonpyrogenic solution administered parenterally by the intravenous or intracardiac (left ventricular chamber) routes, or via endotracheal tube into the bronchial tree. Each milliliter (mL) contains epinephrine 0.1 mg; sodium chloride 8.16 mg; sodium metabisulfite added 0.46 mg; citric acid, anhydrous 2 mg and sodium citrate, dihydrate 0.6 mg added as buffers. May contain additional citric acid and/or sodium citrate for pH adjustment. pH 3.3 (2.2 to 5.0). Epinephrine Injection, USP is oxygen sensitive. The solution contains no bacteriostat or antimicrobial agent and is intended for use only as a single-dose injection. When smaller doses are required the unused portion should be discarded. Epinephrine Injection, USP is a parenteral adrenergic (sympathomimetic) agent and cardiac stimulant. The drug belongs to the group of endogenous compounds known as catecholamines. Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water. Epinephrine, USP is chemically designated 4-[1-hydroxy-2-(methylamino) ethyl]-1, 2 benzenediol, a white, microcrystalline powder. -

Euthanasia Techniques Presented by Wendy Blount, D.V.M
Humongous Insurance Euthanasia Techniques Presented by Wendy Blount, D.V.M. Injection Cephalic Vein 9:23 2 CONFIDENTIAL Injection Other Veins 2:13 3 CONFIDENTIAL Intraperitoneal Injection 7:03 4 CONFIDENTIAL Intracardiac Injection 3:36 5 CONFIDENTIAL Verification of Death 5:22 6 CONFIDENTIAL Euthanasia of Wildlife • Consult with game warden before euthanizing protected species • Assume all rabies vector species to be infected with rabies – Skunk – Bat – Raccoon – Fox, coyote • Send head for rabies examination if any people or domestic animals exposed 7 CONFIDENTIAL Euthanasia of Wildlife • Birds – Intracoelemic injection just below the breast bone – 0.3cc/lb body weight; 3 cc/10 lb body weight • Small Mammals – Rabbits, guinea pigs, mice, hamsters, etc. – Anesthetize, then IP or IC injection – IP without sedation if can be safely restrained 8 CONFIDENTIAL Euthanasia of Wildlife • Reptiles – Must be sedated prior to euthanasia • Especially turtles and tortoises (why?) – Requires supervision of a veterinarian – Know how to identify poisonous snakes – 2-3cc pentobarbital/10 lbs – Reptiles can feign death, and heart rate can fall to a few per minute and still be alive – Death can take a day or two 9 CONFIDENTIAL Euthanasia of Livestock • Gunshot to the head commonly used – May need to follow with shot to the heart • If euthanized with pentobarb, the animal can not be used for food – Wildlife can be poisoned • Cattle in a chute or sedated • Horse euthanasia only by the experienced – 120cc solution attached to an IV line – Horse can rear after injected – Must be clear of the horse when falling 10 CONFIDENTIAL Euthanasia of Livestock • Cattle, sheep and goats – Inject jugular vein • Pigs – Sedate then IC injection – Thick fat layer makes IV injection very difficult, unless the ear vein is used – The squealing is unbearable – Sometimes can hit the ear vein with sedation – Experienced pig handlers can hit the jugular vein, but it takes practice. -

Epinephrine Hydrochloride 595
ASHP INJECTABLE DRUG INFORMATION EPINEPHRINE HYDROCHLORIDE 595 Epinephrine Hydrochloride AHFS 12:12.12 Products Epinephrine hydrochloride is rapidly destroyed by alkalies or oxidizing agents including sodium bicarbonate, halogens, Epinephrine hydrochloride 1 mg/mL is available in 1-mL ampuls, permanganates, chromates, nitrates, nitrites, and salts of easily 30-mL vials, and 0.3-mL auto-injector syringes. Epinephrine reducible metals such as iron, copper, and zinc.4 hydrochloride is also available at a concentration of 0.1 mg/mL (1:10,000) in vials and prefilled syringes. Some products also Visual inspection for color changes may be inadequate to contain sodium chloride, a bisulfite antioxidant, and an antibac- assess compatibility of epinephrine hydrochloride admixtures. terial preservative such as chlorobutanol.1(11/05) 4 In one evaluation with aminophylline stored at 25°C, a color change was not noted until 8 hours had elapsed. However, only pH 40% of the initial epinephrine hydrochloride was still present in From 2.2 to 5.4 17 the admixture at 24 hours.527 Osmolality pH Effects The osmolality of epinephrine hydrochloride (Abbott) 0.1 mg/ The primary determinant of catecholamine stability in intrave- mL was determined to be 273 mOsm/kg by freezing-point nous admixtures is the pH of the solution.527 Epinephrine hydro- depression.1071 A 1-mg/mL solution was determined to have an chloride is unstable in dextrose 5% at a pH above 5.5.48 The pH osmolality of 348 mOsm/kg.1233 of optimum stability is 3 to 4.1072 In one study, the decomposi- tion rate increased twofold (from 5 to 10% in 200 days at 30°C) Trade Name(s) when the pH was increased from 2.5 to 4.5.1259 Adrenalin Chloride, Epipen When lidocaine hydrochloride is mixed with epinephrine hydrochloride, the buffering capacity of the lidocaine hydro- Administration chloride may raise the pH of intravenous admixtures above 5.5, Epinephrine hydrochloride may be administered by subcuta- the maximum necessary for stability of epinephrine hydrochlo- neous, intramuscular, intravenous, or intracardiac injection. -

AVMA Guidelines for the Euthanasia of Animals: 2020 Edition*
AVMA Guidelines for the Euthanasia of Animals: 2020 Edition* Members of the Panel on Euthanasia Steven Leary, DVM, DACLAM (Chair); Fidelis Pharmaceuticals, High Ridge, Missouri Wendy Underwood, DVM (Vice Chair); Indianapolis, Indiana Raymond Anthony, PhD (Ethicist); University of Alaska Anchorage, Anchorage, Alaska Samuel Cartner, DVM, MPH, PhD, DACLAM (Lead, Laboratory Animals Working Group); University of Alabama at Birmingham, Birmingham, Alabama Temple Grandin, PhD (Lead, Physical Methods Working Group); Colorado State University, Fort Collins, Colorado Cheryl Greenacre, DVM, DABVP (Lead, Avian Working Group); University of Tennessee, Knoxville, Tennessee Sharon Gwaltney-Brant, DVM, PhD, DABVT, DABT (Lead, Noninhaled Agents Working Group); Veterinary Information Network, Mahomet, Illinois Mary Ann McCrackin, DVM, PhD, DACVS, DACLAM (Lead, Companion Animals Working Group); University of Georgia, Athens, Georgia Robert Meyer, DVM, DACVAA (Lead, Inhaled Agents Working Group); Mississippi State University, Mississippi State, Mississippi David Miller, DVM, PhD, DACZM, DACAW (Lead, Reptiles, Zoo and Wildlife Working Group); Loveland, Colorado Jan Shearer, DVM, MS, DACAW (Lead, Animals Farmed for Food and Fiber Working Group); Iowa State University, Ames, Iowa Tracy Turner, DVM, MS, DACVS, DACVSMR (Lead, Equine Working Group); Turner Equine Sports Medicine and Surgery, Stillwater, Minnesota Roy Yanong, VMD (Lead, Aquatics Working Group); University of Florida, Ruskin, Florida AVMA Staff Consultants Cia L. Johnson, DVM, MS, MSc; Director,