Unilateral Pulmonary Agenesis, with Esophageal Atresia Long Term Results
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pulmonary Hypoplasia: a Rare Cause of Chronic Cough in TB Endemic Area
Open Journal of Respiratory Diseases, 2019, 9, 18-25 http://www.scirp.org/journal/ojrd ISSN Online: 2163-9418 ISSN Print: 2163-940X Pulmonary Hypoplasia: A Rare Cause of Chronic Cough in TB Endemic Area Ouattara Khadidia1*, Kanoute Tenin1, Baya Bocar1, Soumaré Dianguina1, Kamian Youssouf Mama1, Sidibé Youssouf2, Fofana Aminata3, Traoré Mohamed Maba4, Guindo Ibrahim1, Sidibe Fatoumata1, Dakouo Aimé Paul1, Sanogo Fatoumata Bintou1, Bamba Salimata1, Coulibaly Lamine1, Yossi Oumar1, Kone Drissa Samba1, Toloba Yacouba1 1Department of Pneumology, University Teaching Hospital of Point G, Bamako, Mali 2Department of ENT, Secondary Hospital “Luxembourg”, Bamako, Mali 3Department of ENT, Nianakoro Fomba Hospital, Ségou, Mali 4Department of Radiology, Hospital of Mali, Bamako, Mali How to cite this paper: Khadidia, O., Abstract Tenin, K., Bocar, B., Dianguina, S., Mama, K.Y., Youssouf, S., Aminata, F., Maba, Pulmonary hypoplasia is a rare disease characterized by a defect of lung de- T.M., Ibrahim, G., Fatoumata, S., Paul, velopment more often unilateral. The diagnosis requires several exams to D.A., Bintou, S.F., Salimata, B., Lamine, C., eliminate other causes of pulmonary retraction. We report two cases at the Oumar, Y., Samba, K.D. and Yacouba, T. (2019) Pulmonary Hypoplasia: A Rare department of pneumophtisiology of the University Teaching Hospital of Cause of Chronic Cough in TB Endemic Point G. The first case is a young adult who was complaining of a chronic Area. Open Journal of Respiratory Diseas- cough. Etiological investigation required several exams including spirometry es, 9, 18-25. and Computed tomographic scan (CT scan). After elimination of all sus- https://doi.org/10.4236/ojrd.2019.91002 pected causes of pulmonary opacity, the diagnosis of pulmonary hypoplasia Received: November 30, 2018 was retained. -
The Fetal Care Center at Weill Cornell Medicine
The Fetal Care Center at NewYork-Presbyterian/ Weill Cornell Medicine Prompt and Personalized Care for Women with Complex Pregnancies A Team of Experts At the Fetal Care Center Our multidisciplinary team includes neonatologists (doctors with expertise caring for newborns with birth defects or at NewYork-Presbyterian/ complications associated with prematurity), maternal- fetal medicine specialists (obstetrician/gynecologists with Weill Cornell Medicine, additional training in maternal and fetal complications our experienced team of physicians is of pregnancy), and board-certified pediatric specialists, subspecialists, and pediatric surgeons. dedicated to providing high-quality, state-of-the-art care for you and your Prompt Attention baby. You can rest assured that you will We can make your first appointment quickly, sometimes within 24 business hours of your call. both receive the best possible medical care from the specialists you need Your First Visit in a supportive and compassionate You’ll meet with a neonatologist. We’ll connect you with an MFM specialist or any other doctors you need. environment. Our coordinator can assist you in arranging these appointments. We do our best to schedule as many appointments in the same day as we can, to minimize the number of visits you need to make to our center. We Have A Team of Experts Advanced Imaging the Team Our multidisciplinary team includes neonatologists (doctors We have state-of-the-art MRI capabilities to diagnosis and with expertise caring for newborns with birth defects or clarify complex conditions and help us determine the most You Need complications associated with prematurity), maternal- appropriate treatment options. fetal medicine specialists (obstetrician/gynecologists with additional training in maternal and fetal complications of Delivering Your Baby pregnancy), and board-certifi ed pediatric specialists and If you recently learned your baby subspecialists from every area of surgery and medicine. -

Familial Lung Agenesis Concejo Iglesias P*, Martínez Perez M, Cubero Carralero J, Ocampo Toro WA, and Alvarez Cuenca JH
Case Report iMedPub Journals Medical Case Reports 2020 www.imedpub.com Vol.6 No.2:137 ISSN 2471-8041 DOI: 10.36648/2471-8041.6.2.137 Familial Lung Agenesis Concejo Iglesias P*, Martínez Perez M, Cubero Carralero J, Ocampo Toro WA, and Alvarez Cuenca JH Department of Radiology, Hospital Universitario Severo Ochoa, Madrid, Spain *Corresponding author: Paula Concejo Iglesias, Hospital Universitario Severo Ochoa, Department of Radiology, Avda, De Orellana s/n, Leganés (Madrid) 28911, Spain, Tel: 914818000, E-mail: [email protected] Received date: April 22, 2020; Accepted date: May 22, 2020; Published date: May 28, 2020 Citation: Concejo-Iglesias P, Perez MM, Carralero JC, Toro WAO, Cuenca JHA (2020) Familial Lung Agenesis. Med Case Rep Vol.6 No.2: 137. Abstract Pulmonary agenesis (PA) is a very rare developmental anomaly of the lung. PA involving different members of a family is exceptional. Here, we report two cases of familial left pulmonary agenesis occurred in mother and daughter. Neither of them has other known malformations. Keywords: Pulmonary agenesis; Lung; Congenital disease; Familial disease Introduction Figure 1: PA Chest X-Ray of the mother made at age of 35 Pulmonary agenesis (PA) is a very rare congenital anomaly shows a diffuse opacity in the left hemithorax, mediastinum [1-5] of lung development defined as a complete absence of structures deviated to the left side with compensatory lung tissues, bronchi, and pulmonary vessels [3,6,7]. It may be hyperinflation of the right lung and decreased space uni- or bilateral [1,8] and may be associated with anomalies in between the left ribs. -

Pulmonary Agenesis with Dextrocardia and Hypertrophic
eona f N tal l o B a io n l r o u g y o J Agarwal et al., J Neonatal Biol 2014, 3:3 Journal of Neonatal Biology DOI: 10.4172/2167-0897.1000141 ISSN: 2167-0897 Case Report Open Access Pulmonary Agenesis with Dextrocardia and Hypertrophic Cardiomyopathy: First Case Report Sheetal Agarwal, Arti Maria*, Dinesh Yadav and Narendra Bagri Department of Pediatrics, Ram Manohar Lohia Hospital, New Delhi, India *Corresponding author: Arti Maria, Dept. of Pediatrics, Ram Manohar Lohia Hospital, New Delhi, India, Tel: +919818618586; E-mail: [email protected] Rec date: April 17, 2014, 2014; Acc date: May 23, 2014; Pub date: May 25, 2014 Copyright: © 2014 Agarwal S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Pulmonary agenesis is a rare condition with complete absence of bronchus, lung tissue and vessels. A variety of cardiovascular defects are present in upto 1/3 rd cases of pulmonary agenesis. However, a combination of dextrocardia and hypertrophic cardiomyopathy in association with pulmonary agenesis is not known. Here we report the first case of a neonate presenting with respiratory distress since birth, diagnosed to have hypertrophic cardiomyopathy in association with dextrocardia, multiple cardiac defects and right lung agenesis. Association of heart disease with lung agenesis adversely affects the course and outcome making them a highly lethal association. Keywords: Pulmonary agenesis; Dextrocardia; Hypertrophic compromising cavity size without obstruction of left or right cardiomyopathy; Neonate ventricular outflow tracts. -

A Case of Congenital Syndromic Hydrocephalus: a Subtype of ‘Game-Friedman- Paradice Syndrome'
Oman Medical Journal (2013) Vol. 28, No. 1:63-66 DOI 10. 5001/omj.2013.15 A Case of Congenital Syndromic Hydrocephalus: A Subtype of ‘Game-Friedman- Paradice Syndrome' Tapan Kumar Jana, Hironmoy Roy, Susmita Giri (Jana) Received: 06 Nov 2012 / Accepted: 20 Dec 2012 © OMSB, 2013 Abstract Human hydrocephalus is a disorder of abnormality in CSF flow various other anomalies. The condition was observed first in four or resorption, which has been classified in pertinent literature as offspring from one family and reported by Game K. et al. in 1989. congenital and acquired. Congenital hydrocephalus can present They postulated it to be an autosomal recessive inheritance.8 as an isolated phenomenon which is common; or with associated This syndrome is listed as a "rare disease" by the Office of Rare anomalies affecting other organs, disturbing physiology or presenting Diseases (ORD) of the National Institutes of Health (NIH). This as a syndrome. This report describes a case with congenital foetal means that Game-Friedman-Paradise syndrome, or a subtype hydrocephalus, hypoplastic lungs with super-numery lobations and of Game-Friedman-Paradice syndrome, affects less than one in large left lobe of liver compared to right. Thus far, a review of the 200,000 people in the US population.9 Unfortunately, to date, no literature indicates that this case can be postulated as a subtype of records have been found in the Indian population as searched for. Game-Friedman-Paradice syndrome. Case Report Keywords: Congenital hydrocephalus; Supernumery pulmonary lobations; Game-Friedman-Paradice syndrome. A 21-year-old full term, unbooked primigravida mother was brought in labor emergency in a prolonged first stage of labor. -

Acr–Aser–Scbt-Mr–Spr Practice Parameter for the Performance of Pediatric Computed Tomography (Ct)
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields. The American College of Radiology will periodically define new practice parameters and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service to patients throughout the United States. Existing practice parameters and technical standards will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each practice parameter and technical standard, representing a policy statement by the College, has undergone a thorough consensus process in which it has been subjected to extensive review and approval. The practice parameters and technical standards recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice parameter and technical standard by those entities not providing these services is not authorized. Revised 2019 (Resolution 6) * ACR–ASER–SCBT-MR–SPR PRACTICE PARAMETER FOR THE PERFORMANCE OF PEDIATRIC COMPUTED TOMOGRAPHY (CT) PREAMBLE This document is an educational tool designed to assist practitioners in providing appropriate radiologic care for patients. Practice Parameters and Technical Standards are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care1. -

Agenesis of Lung By
Thorax: first published as 10.1136/thx.13.1.28 on 1 March 1958. Downloaded from Thorax (1958), 13, 28. AGENESIS OF LUNG BY R. ABBEY SMITH AND A. 0. BECH From the King Edward VII Memorial Chest Hospital, Warwick (RECEIVED FOR PUBLICATION JANUARY 6, 1958) Agenesis of a lung is a rare lesion. Reviews of side of the supposed absent lung. The original all reported cases have been published by Hurwitz diagnosis was revised. Per Wexels (1951) de- and Stephens (1937); Deweese and Howard scribed a number of case reports suggestive of (1944); Smart (1946); Per Wexels (1951); agenesis: one of these cases was an infant suffer- Oyamada, Gasul, and Holinger (1953), and Valle ing from congenital atelectasis. (1955). The last author collected and tabulated In older patients it is a not uncommon details of 120 cases. Since Valle's (1955) publica- experience to find identical radiographic and tion, cases have been reported by Warner, Palla- bronchoscopic appearances to those of agenesis, dino, Schwartz, and Schuster (1955); Clark, Scott, a result, for instance, of tuberculous stricture of and Johnson (1955); Hochberg and Naclerio a main bronchus with fibrosis throughout the lung. (1955); Levy (1955); Bariety, Choubrac, Vaudour, Usually some fact in the patient's history or Tupin, and Manouvrier (1955); Sinchez Barrios feature on examination clarifies the diagnosis. and Escobar Aces (1956); Rouco Aja', Codinach, There are patients, however, from whom a history and Segura (1956) ; Chambers and Tancredi (1957); of some acquired cause to account for the clinical and Netterville (1957). A list of references to the findings is unobtainable. -

A Mysterious Paratracheal Mass: Pulmonary Agenesis
Thomas Jefferson University Jefferson Digital Commons Abington Jefferson Health Papers Abington Jefferson Health 6-21-2020 A Mysterious Paratracheal Mass: Pulmonary Agenesis. Qian Zhang Khine S. Shan Follow this and additional works at: https://jdc.jefferson.edu/abingtonfp Part of the Internal Medicine Commons, and the Pulmonology Commons Let us know how access to this document benefits ouy This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Abington Jefferson Health Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Open Access Case Report DOI: 10.7759/cureus.8738 A Mysterious Paratracheal Mass: Pulmonary Agenesis Qian Zhang 1 , Khine S. Shan 2 1. Internal Medicine, Abington Hospital - Jefferson Health, Abington, USA 2. Internal Medicine, University of Maryland Medical Center, Baltimore, USA Corresponding author: Qian Zhang, [email protected] Abstract A 35-year-old lady with a history of possible tuberculosis infection 15 years ago presented to the clinic with the chief complaint of cough. Incidental chest CT showed a right paratracheal and medial right apical heterogeneous soft tissue mass with central areas of calcification that warranted further investigation. -

Posterior Lung Herniation in Pulmonary Agenesis and Aplasia
Brief Research Report | Pediatric Imaging eISSN 2005-8330 https://doi.org/10.3348/kjr.2021.0155 Korean J Radiol 2021;22(10):1690-1696 Posterior Lung Herniation in Pulmonary Agenesis and Aplasia: Chest Radiograph and Cross-Sectional Imaging Correlation Ji Young Kim1, 2, Woo Sun Kim1, 3, 4, Kyung Soo Lee5, Bo-Kyung Je6, Ji Eun Park7, Young Jin Ryu1, 2, Young Hun Choi1, 3, Jung-Eun Cheon1, 3, 4 1Department of Radiology, Seoul National University College of Medicine, Seoul, Korea; 2Department of Radiology, Seoul National University Bundang Hospital, Seongnam, Korea; 3Department of Radiology, Seoul National University Hospital, Seoul, Korea; 4Institute of Radiation Medicine, Seoul National University Medical Research Center, Seoul, Korea; 5Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine (SKKU-SOM), Seoul, Korea; 6Department of Radiology, Korea University College of Medicine, Ansan Hospital, Ansan, Korea; 7Department of Radiology, Ajou University Medical Center, Suwon, Korea Objective: To describe the anatomic locations and imaging features of posterior lung herniation in unilateral pulmonary agenesis and aplasia, focusing on radiograph-CT/MRI correlation. Materials and Methods: A total of 10 patients (seven with pulmonary agenesis and three with pulmonary aplasia, male: female = 1:9, mean age 7.3 years, age range from 1 month to 20 years) were included. Chest radiographs (n = 9), CT (n = 9), and MRI (n = 1) were reviewed to assess the type of lung underdevelopment, presence of anterior and posterior lung herniation, bronchus origin, supplying artery, and draining vein of the herniated lung. Results: Pulmonary agenesis/aplasia more commonly affected the left lung (n = 7) than the right lung (n = 3). -

Unilateral Right Pulmonary Agenesis in Adulthood Radiology Section
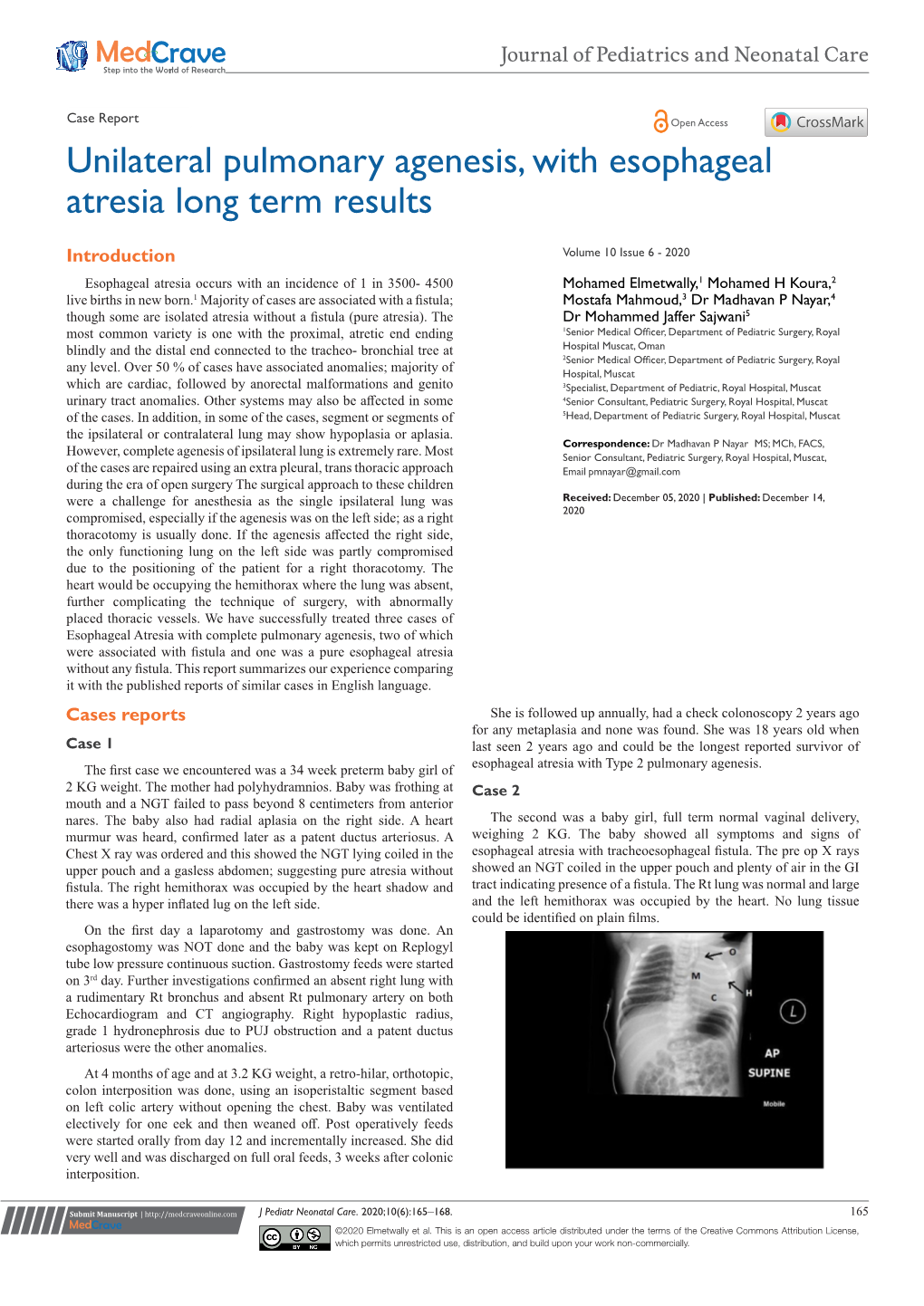
DOI: 10.7860/JCDR/2014/7968.4420 Case Report Unilateral Right Pulmonary Agenesis in Adulthood Radiology Section HEDIYE PINAR GUNBEY1, EMRE GUNBEY 2, ASLI TANRIVERMIS SAYIT3, TANER BULUT4 ABSTRACT Congenital malformations of the lung, which may vary in degrees of severity, are very rare diseases. Pulmonary artery agenesis is a rare anomaly that may occur during the early involution of the proximal portions of the sixth aortic arch, during embryological development of the heart. This agenesis may be accompained by a complete or partial absence of the lung and its bronchus on the same side, which is diagnosed as pulmonary agenesis. In the great majority of the cases, the diagnosis is usually made at or soon after birth and it can be associated with multiple anomalies. However, extremely rare asymptomatic cases may go unnoticed until adulthood. We are presenting a patient with unilateral right pulmonary agenesis, who survived through adulthood without any symptoms and other congenital anomalies. The multislice computed tomography findings and differential diagnoses have been discussed. Keywords: Pulmonary agenesis, Thorax imaging, MSCT CASE REPORT findings, the patient was diagnosed to have right-sided pulmonary A 26-year-old woman was referred to the department of diagnostic agenesis. The mediastinal window setting CT clearly showed Radiology for assessing an incidentally found, unusual view in the deviation of mediastinal structures, heart and trachea totally to the chest radiogram, before she underwent cystectomy of the right right side [Table/Fig-3]. The left lung parenchyma was hyperaerated, ovary. She had a haemorrhagic cyst in the right ovary, that had not with herniating upper lobe connected to the right hemithorax [Table/ regressed for three months. -

CT Features of Lung Agenesis – a Case Series (6 Cases) Jamshid Sadiqi* and Hidayatullah Hamidi
Sadiqi and Hamidi BMC Medical Imaging (2018) 18:37 https://doi.org/10.1186/s12880-018-0281-5 CASEREPORT Open Access CT features of lung agenesis – a case series (6 cases) Jamshid Sadiqi* and Hidayatullah Hamidi Abstract Back ground: Lung agenesis is a rare congenital anomaly. The main etiology of the disease is unknown whereas genetic, iatrogenic and viral factors as well as vitamin A deficiency during early pregnancy may result in developmental failure of primitive lung bud causing unilateral pulmonary agenesis. Affected patients usually present with variable respiratory symptoms and recurrent chest infection at any age. Plain film demonstrates opaque unilateral lung while chest CT scan can definitely diagnosis the disease. The anomaly has three types. Type I is pulmonary agenesis, type II is called pulmonary aplasia and type III is pulmonary hypoplasia. Cases’ presentation: Six patients with main complaint of dyspnea underwent contrast enhanced chest CT in radiology department of French Medical Institute for Mothers and children, Kabul and were diagnosed lung agenesis. Three patients were categorized as type II pulmonary agenesis (aplasia). Two patients, three months old boy and a seven year- old girl demonstrated right lung aplasia. Another patient boy of eighteen years old presented with left lung aplasia. Two boys of four and seven months of age were classified as type I pulmonary agenesis (agenesis). A boy of one year old was diagnosed pulmonary agenesis type III, right lung hypoplasia. Conclusion: Six patients were diagnosed with pulmonary agenesis by Chest CT scan. The clinicians should consider possibility of congenital pulmonary agenesis in dyspneic patients with opaque unilateral hemithorax in plain film. -

Tracheal Stenosis, Pulmonary Agenesis, and Patent Ductus Arteriosus
Thorax: first published as 10.1136/thx.22.1.7 on 1 January 1967. Downloaded from Thorax (1967), 22, 7. Tracheal stenosis, pulmonary agenesis, and patent ductus arteriosus C. S. NELSON, I. K. R. McMILLAN, AND P. K. BHARUCHA From the Wessex Cardiac and Thoracic Centre, Southampton Developmental anomalies of the major pulmonary Through this the child was again bronchoscoped with tree are rare, but are now recognized more fre- some difficulty. The second anomaly was then dis- quently with improved methods of investigation. covered and consisted of an absent left bronchus; the trachea at the expected site of the carina was deviated They are usually associated with other congenital to the left, and at this site the curvature was greatest. defects affecting principally the cardiovascular, A unilateral agenesis of the lung was then thought skeletal, and urogenital systems, but as an isolated most likely, but gross stenosis of the left main lesion they are very rare. Defects of the cardio- bronchus could not be excluded. Further dilatation of vascular system occur more commonly with the stenotic trachea and insertion of a silver tracheo- pulmonary malformations, and these include stomy tube relieved the difficult respiration and patent ductus arteriosus, septal defects, cor bilocu- stridor when the tube had been passed just beyond lare, and maldevelopments of the aortic arch and the stenosis. its great vessels. copyright. A case of congenital tracheal stenosis, unilateral agenesis of the lung, and an associated patent ductus arteriosus is described together with the management. http://thorax.bmj.com/ CASE REPORT Andrew H. was admitted on 22 November 1963 to the Southampton Children's Hospital three weeks after a full-term normal delivery.