An Evaluation of Physico-Chemical Parameters of Tubewell Water from Devrukh City, M.S
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

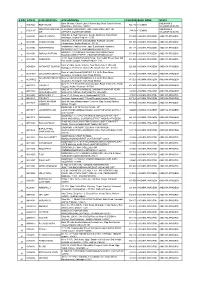
Reg. No Name in Full Residential Address Gender Contact No
Reg. No Name in Full Residential Address Gender Contact No. Email id Remarks 20001 MUDKONDWAR SHRUTIKA HOSPITAL, TAHSIL Male 9420020369 [email protected] RENEWAL UP TO 26/04/2018 PRASHANT NAMDEORAO OFFICE ROAD, AT/P/TAL- GEORAI, 431127 BEED Maharashtra 20002 RADHIKA BABURAJ FLAT NO.10-E, ABAD MAINE Female 9886745848 / [email protected] RENEWAL UP TO 26/04/2018 PLAZA OPP.CMFRI, MARINE 8281300696 DRIVE, KOCHI, KERALA 682018 Kerela 20003 KULKARNI VAISHALI HARISH CHANDRA RESEARCH Female 0532 2274022 / [email protected] RENEWAL UP TO 26/04/2018 MADHUKAR INSTITUTE, CHHATNAG ROAD, 8874709114 JHUSI, ALLAHABAD 211019 ALLAHABAD Uttar Pradesh 20004 BICHU VAISHALI 6, KOLABA HOUSE, BPT OFFICENT Female 022 22182011 / NOT RENEW SHRIRANG QUARTERS, DUMYANE RD., 9819791683 COLABA 400005 MUMBAI Maharashtra 20005 DOSHI DOLLY MAHENDRA 7-A, PUTLIBAI BHAVAN, ZAVER Female 9892399719 [email protected] RENEWAL UP TO 26/04/2018 ROAD, MULUND (W) 400080 MUMBAI Maharashtra 20006 PRABHU SAYALI GAJANAN F1,CHINTAMANI PLAZA, KUDAL Female 02362 223223 / [email protected] RENEWAL UP TO 26/04/2018 OPP POLICE STATION,MAIN ROAD 9422434365 KUDAL 416520 SINDHUDURG Maharashtra 20007 RUKADIKAR WAHEEDA 385/B, ALISHAN BUILDING, Female 9890346988 DR.NAUSHAD.INAMDAR@GMA RENEWAL UP TO 26/04/2018 BABASAHEB MHAISAL VES, PANCHIL NAGAR, IL.COM MEHDHE PLOT- 13, MIRAJ 416410 SANGLI Maharashtra 20008 GHORPADE TEJAL A-7 / A-8, SHIVSHAKTI APT., Male 02312650525 / NOT RENEW CHANDRAHAS GIANT HOUSE, SARLAKSHAN 9226377667 PARK KOLHAPUR Maharashtra 20009 JAIN MAMTA -

KONKAN TALENT SEARCH EXAMINATION - 2016 Schoolwise Student's List
KONKAN TALENT SEARCH EXAMINATION - 2016 Schoolwise Student's List School Name : PATIL HIGH SCHOOL, POYNAD School Code : IAL001 Center Name : CHINTAMANRAO KELKAR VIDYALAYA, ALIBAG Center Code : I15 Sr. No. SeatNo Name Gender Class Medium 1 1041 KARVE ACHAL SUDHIR F 8 MARATHI 2 1042 PATIL OMKAR RAJENDRA M 8 MARATHI 3 1043 PATIL VIDHITA GAJANAN F 8 MARATHI 4 1044 SHINDE PIYUSH KISHOR M 8 MARATHI 5 1666 GHARAT RAJ PRASAD M 8 MARATHI 6 1667 KUTHE SUMIT PRAMOD M 8 MARATHI 7 1668 PATIL SANIKA RAVINDRA F 8 MARATHI 8 1669 PINGLE TANISHA JAGDISH F 8 MARATHI Class --> 8th 9th Total No. of Students--> 8 0 8 Page 1 of 1 KONKAN TALENT SEARCH EXAMINATION - 2016 Schoolwise Student's List School Name : CHINTAMANRAO KELKAR VIDYALAYA, ALIBAG School Code : IAL010 Center Name : CHINTAMANRAO KELKAR VIDYALAYA, ALIBAG Center Code : I15 Sr. No. SeatNo Name Gender Class Medium 1 1105 ANGRE BHUSHAN SANJAY M 8 SEMI-ENGLISH 2 1106 BHAGAT BHAVIK GOPAL M 8 SEMI-ENGLISH 3 1107 DHUMAL ARYA DIPAK M 8 SEMI-ENGLISH 4 1108 JAGDALE SAKSHI ROHIDAS F 8 SEMI-ENGLISH 5 1109 KUMBHAR JAY SHASHIKANT M 8 SEMI-ENGLISH 6 1110 MAHADIK DIKSHITA SANJAY F 8 SEMI-ENGLISH 7 1111 NAVARE DIKSHA DILIP F 8 SEMI-ENGLISH 8 1112 PATIL SANIKA SANTOSH F 8 SEMI-ENGLISH 9 1113 PATIL VEDIKA VIJAY F 8 SEMI-ENGLISH 10 1114 RAUT RUTUJA RAJAN F 8 SEMI-ENGLISH 11 1115 SHINDE YAMINI JAYENDRA F 8 SEMI-ENGLISH 12 1116 SUNKALE MRUNMAYI RAJENDRA F 8 SEMI-ENGLISH 13 1117 THAKUR VEDANGI VILAS F 8 SEMI-ENGLISH 14 1118 VARTAK OM RAJESH M 8 SEMI-ENGLISH 15 1119 WAGHMARE PARTH JAYENDRA M 8 SEMI-ENGLISH 16 1120 GHADGE -

(2015) Shivaji University, Kolhapur
Shivaji University, Kolhapur Register of Registered Graduate (2015) Faculty of ENGINEERING & TECHNOLOGY Registration No. 89801 Registration No. 89809 1 Shri. ABHYANKAR GANGADHAR LAXMAN 9 Shri. ADHYAPAK ANIL AMRIT SHIVAJI NAGAR 108 KRISHNA NIVAS A/P - KUNDGOL 4TH LANE HINDU COLONY DADAR T.T. MUMBAI - 14 DD Dist. DHARWAR Dist. MUMBAI Registration No. 89802 Registration No. 89810 2 Shri. ABHYANKAR GANGADHAR SHANKAR 10 Shri. ADKAR ARUN RAMACHANDRA 40 PRERANA TILAK NAGAR 382 KASBA BARSI V.P.ROAD MUMBAI - 4 Dist. SOLAPUR Dist. MUMBAI Registration No. 89803 Registration No. 89811 3 Shri. ABHYANKAR HEMANT KESHAV 11 Shri. ADKAR SHASHIKANT GOVIND 977 A SATTIKAR LANE 231 NAVI PETH GAON-BHAG JALGAON SANGLI Dist. SANGLI Dist. JALGAON Registration No. 89804 Registration No. 89812 4 Shri. ABHYANKAR PURUSHOTTAM SITARAM 12 Shri. ADMUTHE SUNIL SHANTINATH PATWARDHAN BLOCKS DEPARTMENT OF INSTRUMENTION NEAR WALCHAND COLLEGE OF ENGINEERING P V P INSTITUTE OF TECHNOLOGY VISHRAM BAUG SANGLI BUDHGAON Dist. SANGLI Dist. SANGLI Registration No. 89805 Registration No. 89813 5 Shri. ABHYANKAR SURESH SHRIDHAR 13 Shri. ADNAIK PRAKASH MOHANRAO C/O - S.W.ABHYANKAR 1877/B FLAT NO 2 AMRAPALI KHAPARDE GARDEN APT RAJARAMPURI 8TH LANE AMARAVATI (VIDARBH) KOLHAPUR Dist. AMARAVATI Dist. KOLHAPUR 416008 Registration No. 89806 Registration No. 89814 6 Shri. ADADANDE AKSHAYKUMAR BALASAHEB 14 Shri. ADSUL AKSHAY RAMCHANDRA KARVEER NAGAR HOUSING SOCIETY, PLOT NO 27 B, 1014/29 SAI COLONY AIRPORT ROAD, UJALAIWADI KOLHAPUR APTE NAGAR, KOLHAPUR 416004 Dist. KOLHAPUR Dist. KOLHAPUR Registration No. 89807 Registration No. 89815 7 Shri. ADANI KRISHNAKUMAR DHARAMSHI 15 Shri. ADURE GAUTAM SURESH R.NO. 96 PL NO 45 B/1 LAYOUT NO 1 GITA GRAHA 4 PICKET ROAD BABA JARAG NAGAR MUMBAI - 2 KOLHAPUR - 416007 Dist. -

Annexure-V State/Circle Wise List of Post Offices Modernised/Upgraded
State/Circle wise list of Post Offices modernised/upgraded for Automatic Teller Machine (ATM) Annexure-V Sl No. State/UT Circle Office Regional Office Divisional Office Name of Operational Post Office ATMs Pin 1 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA PRAKASAM Addanki SO 523201 2 Andhra Pradesh ANDHRA PRADESH KURNOOL KURNOOL Adoni H.O 518301 3 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM AMALAPURAM Amalapuram H.O 533201 4 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Anantapur H.O 515001 5 Andhra Pradesh ANDHRA PRADESH Vijayawada Machilipatnam Avanigadda H.O 521121 6 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA TENALI Bapatla H.O 522101 7 Andhra Pradesh ANDHRA PRADESH Vijayawada Bhimavaram Bhimavaram H.O 534201 8 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA VIJAYAWADA Buckinghampet H.O 520002 9 Andhra Pradesh ANDHRA PRADESH KURNOOL TIRUPATI Chandragiri H.O 517101 10 Andhra Pradesh ANDHRA PRADESH Vijayawada Prakasam Chirala H.O 523155 11 Andhra Pradesh ANDHRA PRADESH KURNOOL CHITTOOR Chittoor H.O 517001 12 Andhra Pradesh ANDHRA PRADESH KURNOOL CUDDAPAH Cuddapah H.O 516001 13 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM VISAKHAPATNAM Dabagardens S.O 530020 14 Andhra Pradesh ANDHRA PRADESH KURNOOL HINDUPUR Dharmavaram H.O 515671 15 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA ELURU Eluru H.O 534001 16 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudivada Gudivada H.O 521301 17 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudur Gudur H.O 524101 18 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Guntakal H.O 515801 19 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA -

Geoelectrical Investigations for Potential Groundwater in Parts of Ratnagiri and Kolhapur Districts, Maharashtra
I J R B A T, Vol. V, Special Issue (3), Nov-2017 ISSN 2347 – 517X INTERNATIONAL JOURNAL OF RESEARCHES IN BIOSCIENCES, AGRICULTURE AND TECHNOLOGY © VISHWASHANTI MULTIPURPOSE SOCIETY (Global Peace Multipurpose Society) R. No. MH-659/13(N) www.vmsindia.org GEOELECTRICAL INVESTIGATIONS FOR POTENTIAL GROUNDWATER IN PARTS OF RATNAGIRI AND KOLHAPUR DISTRICTS, MAHARASHTRA Mahendra R. Gaikwad and Gautam Gupta 1* Department of Geology, G.B. Tatha Tatyasaheb Khare Commerce, Parvatibai Gurupad Dhere Art’s and Shri. Mahesh Janardan Bhosale Science College, Guhagar Dist. Ratnagiri - 415703 (M.S.), (India) 2 Indian Institute of Geomagnetism, New Panvel (W), Navi Mumbai - 410 218 [email protected] Abstract Electrical resistivity is one of the most effective ge ophysical methods for investigating the presence of groundwater. In a hard rock terrain like the Kolhapur, Ratnagiri and adjoining regions in the Deccan Volcanic Province of Maharashtra, the nature and extent of we athering may vary significantly, depending mostly on the presence of fractures and lineaments at depth and the geomorphological features at the surface. Hence , electrical resistivity studies are vital in a hard rock terrain for identification and analysis of concealed lineaments and fractures zones. An attempt is made here to understand the tectonic framework of over Devrukh-Ganapatipule region and Malkapur- Sakarpa-Ratnagiri region and its relation to the movement of groundwater. A total of 43 vertical electrical soundings we re conducted in Devrukh-Ganapatipule and Malkapur-Sakarpa-Ratnagiri region. The profile over the Malkapur- Ratnagiri area was divided into two profiles. It is observed from the two dimensional geoelectric cross-section that this region has more potential groundwater aquifers than the previous profile. -
S No Atm Id Atm Location Atm Address Pincode Bank
S NO ATM ID ATM LOCATION ATM ADDRESS PINCODE BANK ZONE STATE Bank Of India, Church Lane, Phoenix Bay, Near Carmel School, ANDAMAN & ACE9022 PORT BLAIR 744 101 CHENNAI 1 Ward No.6, Port Blair - 744101 NICOBAR ISLANDS DOLYGUNJ,PORTBL ATR ROAD, PHARGOAN, DOLYGUNJ POST,OPP TO ANDAMAN & CCE8137 744103 CHENNAI 2 AIR AIRPORT, SOUTH ANDAMAN NICOBAR ISLANDS Shop No :2, Near Sai Xerox, Beside Medinova, Rajiv Road, AAX8001 ANANTHAPURA 515 001 ANDHRA PRADESH ANDHRA PRADESH 3 Anathapur, Andhra Pradesh - 5155 Shop No 2, Ammanna Setty Building, Kothavur Junction, ACV8001 CHODAVARAM 531 036 ANDHRA PRADESH ANDHRA PRADESH 4 Chodavaram, Andhra Pradesh - 53136 kiranashop 5 road junction ,opp. Sudarshana mandiram, ACV8002 NARSIPATNAM 531 116 ANDHRA PRADESH ANDHRA PRADESH 5 Narsipatnam 531116 visakhapatnam (dist)-531116 DO.NO 11-183,GOPALA PATNAM, MAIN ROAD NEAR ACV8003 GOPALA PATNAM 530 047 ANDHRA PRADESH ANDHRA PRADESH 6 NOOKALAMMA TEMPLE, VISAKHAPATNAM-530047 4-493, Near Bharat Petroliam Pump, Koti Reddy Street, Near Old ACY8001 CUDDAPPA 516 001 ANDHRA PRADESH ANDHRA PRADESH 7 Bus stand Cudappa, Andhra Pradesh- 5161 Bank of India, Guntur Branch, Door No.5-25-521, Main Rd, AGN9001 KOTHAPET GUNTUR 522 001 ANDHRA PRADESH ANDHRA PRADESH Kothapeta, P.B.No.66, Guntur (P), Dist.Guntur, AP - 522001. 8 Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW8001 GAJUWAKA BRANCH 530 026 ANDHRA PRADESH ANDHRA PRADESH 9 Gajuwaka, Anakapalle Main Road-530026 GAJUWAKA BRANCH Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW9002 530 026 ANDHRA PRADESH ANDHRA PRADESH -

State District Branch Address Centre Ifsc Contact1 Contact2 Contact3 Micr Code
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE ANDAMAN NO 26. MG ROAD AND ABERDEEN BAZAR , NICOBAR PORT BLAIR -744101 704412829 704412829 ISLAND ANDAMAN PORT BLAIR ,A & N ISLANDS PORT BLAIR IBKL0001498 8 7044128298 8 744259002 UPPER GROUND FLOOR, #6-5-83/1, ANIL ANIL NEW BUS STAND KUMAR KUMAR ANDHRA ROAD, BHUKTAPUR, 897889900 ANIL KUMAR 897889900 PRADESH ADILABAD ADILABAD ADILABAD 504001 ADILABAD IBKL0001090 1 8978899001 1 1ST FLOOR, 14- 309,SREERAM ENCLAVE,RAILWAY FEDDER ROADANANTAPURA ANDHRA NANTAPURANDHRA ANANTAPU 08554- PRADESH ANANTAPUR ANANTAPUR PRADESH R IBKL0000208 270244 D.NO.16-376,MARKET STREET,OPPOSITE CHURCH,DHARMAVA RAM- 091 ANDHRA 515671,ANANTAPUR DHARMAVA 949497979 PRADESH ANANTAPUR DHARMAVARAM DISTRICT RAM IBKL0001795 7 515259202 SRINIVASA SRINIVASA IDBI BANK LTD, 10- RAO RAO 43, BESIDE SURESH MYLAPALL SRINIVASA MYLAPALL MEDICALS, RAILWAY I - RAO I - ANDHRA STATION ROAD, +91967670 MYLAPALLI - +91967670 PRADESH ANANTAPUR GUNTAKAL GUNTAKAL - 515801 GUNTAKAL IBKL0001091 6655 +919676706655 6655 18-1-138, M.F.ROAD, AJACENT TO ING VYSYA BANK, HINDUPUR , ANANTAPUR DIST - 994973715 ANDHRA PIN:515 201 9/98497191 PRADESH ANANTAPUR HINDUPUR ANDHRA PRADESH HINDUPUR IBKL0001162 17 515259102 AGRICULTURE MARKET COMMITTEE, ANANTAPUR ROAD, TADIPATRI, 085582264 ANANTAPUR DIST 40 ANDHRA PIN : 515411 /903226789 PRADESH ANANTAPUR TADIPATRI ANDHRA PRADESH TADPATRI IBKL0001163 2 515259402 BUKARAYASUNDARA M MANDAL,NEAR HP GAS FILLING 91 ANDHRA STATION,ANANTHAP ANANTAPU 929710487 PRADESH ANANTAPUR VADIYAMPETA UR -

Download Third Cycle
Self-Study Report – Third Cycle - 2015 Devrukh Shikshan Prasarak Mandal's Nya. Tatyasaheb Athalye Arts, Ved. S.R. Sapre Commerce and Vid. Dadasaheb Pitre Science College, Devrukh, Dist – Ratangiri (Maharashtra) - 415 804 Vision: To provide higher education and job based skills to the youth of Sangmeshwar tehsil at minimal cost. Mission: Imparting higher education and inculcating sense of civic responsibilities with the aim of community development. Objectives: To facilitate Higher Education irrespective of caste, creed, race and religion. To improve and develop personality of the students by imparting education and skills with special emphasis on socially, economically, educationally disadvantaged and downtrodden students. To inculcate civic responsibilities among the students through the organization of various activities. To create awareness among public about higher education by arranging lectures, publishing annual magazine, organizing camps, etc. NAAC Re-Accreditation - Third Cycle 2010 -- 2015 Steering Committee 1. Principal Dr. Narendra P. Tendolkar : Chair Person 2. Mr. Arvind M. Kulkarni : Steering Committee Coordinator 3. Dr. Sardar A. Patil : Member 4. Dr. Meera R. Kale : Member 5. Dr. Pratap V. Naikwade : Member 6. Mr. Uday B. Bhatye : Member 7. Mr. Vivek S. Bhopatkar : Office Superintendent Athalye-Sapre-Pitre college, Devrukh, Maharashtra–415804 Self-Study Report – Third Cycle - 2015 Preface It is indeed a great pleasure to submit the Reaccreditation and Assessment Report (RAR) for the 3rd cycle. It was impossible to present this report, replete with developmental measures and activities undertaken during the period of 3rd accreditation, without the commendable and active support and trust of our parent institution in all our decisions and actions. Hence we seize this opportunity to write about our parent institution. -

SOUVENIR Geography National Conference 2011
UGC Sponsored Three Day National Conference On Population, Environment and Tourism UGC Sponsored Three Day National Conference On Population, Environment and Tourism March 3-5, 2011 (Thursday, Friday and Saturday) Organized by Devrukh Shikshan Prasasrak Mandal’s Nya. Tatyasaheb Athalye Arts, Ved.S.R.Sapre Commerce and Vid. Dadasaheb Pitre Science College Devrukh, Dist. Ratnagiri, Maharashtra (India) (Reaccredited with B Grade (CGPA 2.82) (Mumbai University Best College Award) Our Patron Hon. Mr. V. M. Pitre, President, Devrukh Shikshan Prasarak Mandal, Devrukh, Dist. Ratnagiri Convener Dr. N. P. Tendolkar Principal, A. S. P. College, Devrukh Organizing Secretary Dr. Sardar A. Patil Assistant Professor, Department of Geography, A. S. P. College, Devrukh Email: [email protected] [email protected] Nya. Tatyasaheb Athalye Arts, Ved.S.R.Sapre Commerce and Vid. Dadasaheb Pitre Science College Devrukh, Dist. Ratnagiri, Maharashtra 1 UGC Sponsored Three Day National Conference On Population, Environment and Tourism Focal Theme Population, Environment and Tourism Sub–Themes Changing population characteristics. Population Growth and Development Problems of Population and Human Development Index Population Growth, Distribution, Sex Ratio, Literacy and Status Social well being Environment Degradation and its Conservation Environmental Hazards and its Management Environment and Agriculture Role of Tourism and Ecotourism in the Economic Development Application of Remote Sensing, GIS and GPS in the Study of Population, Environment and Tourism. All Allied themes. Nya. Tatyasaheb Athalye Arts, Ved.S.R.Sapre Commerce and Vid. Dadasaheb Pitre Science College Devrukh, Dist. Ratnagiri, Maharashtra 2 UGC Sponsored Three Day National Conference On Population, Environment and Tourism Foreword Hon. Shri. V. M. Pitre President, D. -

Unemployment and out Migration in Ratnagiri District
RESEARCH PAPER Economics Volume : 6 | Issue : 5 | May 2016 | ISSN - 2249-555X | IF : 3.919 | IC Value : 74.50 Unemployment and Out Migration in Ratnagiri District KEYWORDS Unemployment, Out migration. RAHUL V. THOKE Assistant Professor in Economics , Athalye -Sapre - Pitre College, Devrukh, Dist- Ratnagiri . ABSTRACT This research paper is based on Minor Reasearch Project sanctioned by UGC.Study area of this paper is Ratnagiri district.To draw the sample from Ratnagiri district, a multi stage sampling technique was used. To show relationship between unemployment and out migrationion X2 text was used.The present study is based on primary and secondary data. Survey and Interview methods were used to collect primary data from the sample. The proportion of workers in manufacturing is extremely low in Ratnagiri.It shows less industrial development.Ratna- giri is rural district it means near about 3/4 of population live in rural area. Higher sex ratio may indicates a very large out migration of male population from rural districts (Ratnagiri) to the metro politant city of Mumbai ,to other parts of India and even abroad. Lact of employment opportunities is the main cause of migration from rural to urban area. Introduction: - • Centre for development studies, Trivandrum, Kerala. Ratnagiri is a economically backward district. There is no Above mention all research centers and organizations have industrial as well as agricultural development. There are published their research. that literature has been reviewed. many constrains viz. natural, economical, political, etc. Therefore unemployed persons have to migrate for job. Area of Research –(sample design) Mostly people from this district prefer to go Mumbai or In order to draw the sample from Ratnagiri district, a mul- Pune for getting job. -

Handbook-Code of Conduct
The Prabodhan Shikshan Prasarak Sanstha’s Rajendra Mane College of Engineering and Technology Ambav (Devrukh) The Prabodhan Shikshan Prasarak Sanstha’s Rajendra Mane College of Engineering and Technology Ambav (Devrukh) CODE BOOK CODE BOOK The Prabodhan Shikshan Prasarak Sanstha’s Rajendra Mane College of Engineering and Technology Ambav (Devrukh) The Prabodhan Shikshan Prasarak Sanstha’s Rajendra Mane College of Engineering and Technology Ambav (Devrukh) About Sanstha Prabodhan Shikshan Prasarak Sanstha is a registered Public Charitable Trust registered under the Public Trust act 1950 (Regn. No. E-697 Ratnagiri, dated 7th June 1995). The Trust has been carrying on its activities for last two decades mainly in the field of education. It is the able chairmanship of Hon. Shri. Ravindraji Mane (Former Minister of State, Government of Maharashtra) who laid down the foundation stone of Rajendra Mane College of Engineering and Technology at Ambav (Devrukh) in 1998. The college is approved by AICTE, New Delhi, recognized by Directorate of Technical Education, Maharashtra and affiliated to University of Mumbai. RMCET is one of the premier colleges which has created its niche in Konkan region. The 31.15 acres area of the college campus is situated at Ambav (Devrukh), Ratnagiri district surrounded by lush green hills of Sahyadri (Western Ghat) ranges and is a temple for technical education About Institute The Prabodhan Shikshan Prasarak Sanstha’s Rajendra Mane College of Engineering and Technology, Ambav was established in the year 1998, with the sole objective to promote education for the people of the Konkan region and also work for the welfare and upliftment of society. -

Pdf/2003-2Pp94-102.Pdf 2
Vol 1 Issue 11 May 2012 ISSN No :2231-5063 InternationaORIGINALl M ARTICLEultidisciplinary Research Journal Golden Research Thoughts Chief Editor Dr.Tukaram Narayan Shinde Associate Editor Publisher Dr.Rajani Dalvi Mrs.Laxmi Ashok Yakkaldevi Honorary Mr.Ashok Yakkaldevi Welcome to GRT RNI MAHMUL/2011/38595 ISSN No.2231-5063 Golden Research Thoughts Journal is a multidisciplinary research journal, published monthly in English, Hindi & Marathi Language. All research papers submitted to the journal will be double - blind peer reviewed referred by members of the editorial board.Readers will include investigator in universities, research institutes government and industry with research interest in the general subjects. Regional Editor Manichander Thammishetty Ph.d Research Scholar, Faculty of Education IASE, Osmania University, Hyderabad International Advisory Board Kamani Perera Mohammad Hailat Hasan Baktir Regional Center For Strategic Studies, Sri Dept. of Mathematical Sciences, English Language and Literature Lanka University of South Carolina Aiken Department, Kayseri Janaki Sinnasamy Abdullah Sabbagh Ghayoor Abbas Chotana Librarian, University of Malaya Engineering Studies, Sydney Dept of Chemistry, Lahore University of Management Sciences[PK] Romona Mihaila Ecaterina Patrascu Spiru Haret University, Romania Spiru Haret University, Bucharest Anna Maria Constantinovici AL. I. Cuza University, Romania Delia Serbescu Loredana Bosca Spiru Haret University, Bucharest, Spiru Haret University, Romania Ilie Pintea, Romania Spiru Haret University, Romania Fabricio Moraes de Almeida Anurag Misra Federal University of Rondonia, Brazil Xiaohua Yang DBS College, Kanpur PhD, USA George - Calin SERITAN Titus PopPhD, Partium Christian Faculty of Philosophy and Socio-Political ......More University, Oradea,Romania Sciences Al. I. Cuza University, Iasi Editorial Board Pratap Vyamktrao Naikwade Iresh Swami Rajendra Shendge ASP College Devrukh,Ratnagiri,MS India Ex - VC.