Ground Water Quality Analysis of Villages in Gulbarga District
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Content Page No's 1-5 6 6 7 8 9 10-12 13-50 51-52 53-82 83-93
Table of Content Executive summary Page No’s i. Introduction 1-5 ii. Background 6 iii. Vision 6 iv. Objective 7 V. Strategy /approach 8 VI. Rationale/ Justification Statement 9 Chapter-I: General Information of the District 1.1 District Profile 10-12 1.2 Demography 13-50 1.3 Biomass and Livestock 51-52 1.4 Agro-Ecology, Climate, Hydrology and Topography 53-82 1.5 Soil Profile 83-93 1.6 Soil Erosion and Runoff Status 94 1.7 Land Use Pattern 95-139 Chapter II: District Water Profile: 2.1 Area Wise, Crop Wise irrigation Status 140-150 2.2 Production and Productivity of Major Crops 151-158 2.3 Irrigation based classification: gross irrigated area, net irrigated area, area under protective 159-160 irrigation, un irrigated or totally rain fed area Chapter III: Water Availability: 3.1: Status of Water Availability 161-163 3.2: Status of Ground Water Availability 164-169 3.3: Status of Command Area 170-194 3.4: Existing Type of Irrigation 195-198 Chapter IV: Water Requirement /Demand 4.1: Domestic Water Demand 199-200 4.2: Crop Water Demand 201-210 4.3: Livestock Water Demand 211-212 4.4: Industrial Water Demand 213-215 4.5: Water Demand for Power Generation 216 4.6: Total Water Demand of the District for Various sectors 217-218 4.7: Water Budget 219-220 Chapter V: Strategic Action Plan for Irrigation in District under PMKSY 221-338 List of Tables Table 1.1: District Profile Table 1.2: Demography Table 1.3: Biomass and Live stocks Table 1.4: Agro-Ecology, Climate, Hydrology and Topography Table 1.5: Soil Profile Table 1.7: Land Use Pattern Table -

HŒ臬 A„簧綟糜恥sµ, Vw笑n® 22.12.2019 Š U拳 W
||Om Shri Manjunathaya Namah || Shri Kshethra Dhamasthala Rural Development Project B.C. Trust ® Head Office Dharmasthala HŒ¯å A„®ãtÁS®¢Sµ, vw¯ºN® 22.12.2019 Š®0u®± w®lµu® îµ±°ªæX¯Š®N®/ N®Zµ°‹ š®œ¯‡®±N®/w®S®u®± š®œ¯‡®±N® œ®±uµÛ‡®± wµ°Š® wµ°î®±N¯r‡®± ªRq® y®‹°£µ‡®± y®ªq¯ºý® D Nµ¡®w®ºruµ. Cu®Š®ªå 50 î®±q®±Ù 50 Oʺq® œµX®±Ï AºN® y®lµu®î®Š®w®±Ý (¬šµ¶g¬w®ªå r¢›Š®±î®ºqµ N®Zµ°‹/w®S®u®± š®œ¯‡®±N® œ®±uµÛSµ N®xÇ®Õ ïu¯ãœ®Áqµ y®u®ï î®±q®±Ù ®±š®±é 01.12.2019 NµÊ Aw®æ‡®±î¯S®±î®ºqµ 25 î®Ç®Á ï±°Š®u®ºqµ î®±q®±Ù îµ±ªæX¯Š®N® œ®±uµÛSµ N®xÇ®Õ Hš¬.Hš¬.HŒ¬.› /z.‡®±±.› ïu¯ãœ®Áqµ‡µ²ºvSµ 3 î®Ç®Áu® Nµ©š®u® Aw®±„Â®î® î®±q®±Ù ®±š®±é 01.12.2019 NµÊ Aw®æ‡®±î¯S®±î®ºqµ 30 î®Ç®Á ï±°Š®u®ºqµ ) î®±±ºvw® œ®ºq®u® š®ºu®ý®Áw®NµÊ B‡µ±Ê ¯l®Œ¯S®±î®¼u®±. š®ºu®ý®Áw®u® š®Ú¡® î®±q®±Ù vw¯ºN®î®w®±Ý y®äqµã°N®î¯T Hš¬.Hº.Hš¬ î®±²©N® ¯Ÿr x°l®Œ¯S®±î®¼u®±. œ¯cŠ¯u® HŒ¯å A„®ãtÁS®¢Sµ A†Ãw®ºu®wµS®¡®±. Written test Sl No Name Address Taluk District mark Exam Centre out off 100 11 th ward near police station 1 A Ashwini Hospete Bellary 33 Bellary kampli 2 Abbana Durugappa Nanyapura HB hally Bellary 53 Bellary 'Sri Devi Krupa ' B.S.N.L 2nd 3 Abha Shrutee stage, Near RTO, Satyamangala, Hassan Hassan 42 Hassan Hassan. -

Name of the Village
POPULATION PROFILE OF KALBURUGI Dist AS PER 2011 CENSUS Total SC ST Sl No Name of the Village % % Population Population Population 1 Gulbarga 2566326 648782 25.28 65259 2.54 2 Gulbarga 1730775 489697 28.29 50074 2.89 3 Gulbarga 835551 159085 19.04 15185 1.82 4 Aland 342207 85516 24.99 6843 2.00 5 Aland 299836 79777 26.61 6508 2.17 6 Aland 42371 5739 13.54 335 0.79 7 Jamga Khandala 1215 756 62.22 30 2.47 8 Tadola 2568 716 27.88 1 0.04 9 Alanga 2571 281 10.93 7 0.27 10 Sirur (G) 1378 240 17.42 63 4.57 11 Gadlegaon 686 250 36.44 0 0.00 12 Khajuri 6744 912 13.52 9 0.13 13 Jawalga (J) 1707 542 31.75 57 3.34 14 Tugaon 837 222 26.52 27 3.23 15 Annur 1248 295 23.64 69 5.53 16 Hodlur 2697 361 13.39 4 0.15 17 Subhashnagar 1057 1055 99.81 1 0.09 18 Nandgur 500 152 30.40 0 0.00 19 Kotanhipperga 1699 178 10.48 0 0.00 20 Jamga Ruderwadi 1766 502 28.43 0 0.00 21 Rudrawadi 3004 699 23.27 57 1.90 22 Bableshwar 884 228 25.79 65 7.35 23 Bangerga 2334 662 28.36 55 2.36 24 Khandala 1434 528 36.82 141 9.83 25 Nirgudi 3109 307 9.87 87 2.80 26 Matki 4206 732 17.40 291 6.92 27 Teerth 1636 315 19.25 12 0.73 28 Salegaon 2342 506 21.61 135 5.76 29 Chitali 1511 237 15.68 336 22.24 30 Kinnisultan 3322 745 22.43 1 0.03 31 Kanmas 1254 348 27.75 3 0.24 32 Bharked 865 97 11.21 39 4.51 33 Sangunda 1097 279 25.43 4 0.36 34 Belamogi 5127 1613 31.46 189 3.69 35 Karhari 1584 567 35.80 0 0.00 36 Salgera (V.K.) 5182 1734 33.46 137 2.64 37 Lengthi 1956 457 23.36 354 18.10 38 Ladmugli 2636 575 21.81 2 0.08 39 Kalkutga 691 184 26.63 56 8.10 40 Kudmud 1610 806 50.06 0 0.00 41 Ambalga -

Remarks Afzalpur Page 1 of 55 04/04/2019
List of Cancellation of Polling Duty S. No. Letter No. Name Designation Department Emp. ID S/W/DO Reason for cancellation Office Class remarks Rehearsal Centre Code: 1 Assembly Segment under which centre falls Afzalpur 139750 AMARNATH DHULE ASSISTANT ENGINEER PW-PUBLIC WORKS 1 17004700020002 DEPARTMENT Cancelled by Committee Marriage PRO KUPENDRA DHULE Office of the Executive Engineer, PWP & IWTD ,Division Old Jewargi 143801 SIDRAMAPPA B WALIKAR SERICULTURE INSPECTOR SE-COMMISSIONER FOR 2 17005900020012 SERICULTURE DEVELOPMENT SST TEAM IN AFZALPUR PRO BHIMASYA WALIKAR DEPUTY DIRECTOR OF SERICULTURE 144462 DR SHAKERA TANVEER ASSISTANT PROFESSOR EC-DEPARTMENT OF 3 17001400080012 COLLEGIATE EDUCATION DOUBLE ORDERS PRO MOHAMMED JAMEEL AHMED Government First Grade College Afzalpur 144467 DR MALLIKARJUN M SAVARKAR ASSISTANT PROFESSOR EC-DEPARTMENT OF 4 17001400090005 COLLEGIATE EDUCATION EVM NODAL OFFICER IN PRO MADARAPPA AFZALPUR Govt First Grade Colloge Karajagi Tq Afzalpur Dist Gulbarga 144476 NAVYA N LECTURER ET-DEPARTMENT OF 5 17001800010067 TECHNICAL EDUCATION ON MATERNITY LEAVE PRO NARASIMHAREDDY B LECTURER SELECTION GRADE 144569 JALEEL KHAN JUNIOR ENGINEER MR-DEPARTMENT OF MINOR 6 17003800020022 IRRIGATION SECTOR OFFICER IN AFZALPUR PRO OSMAN KHAN Assistant Executive Engineer 144813 HUMERA THASEEN TRAINED GRADUATE TEACHER QE-3201QE0001-BEO AFZALPUR 7 17008300090006 (TGT) Cancelled by Committee UMRAH PRO M A RASHEED TOUR. BLOCK EDUCATIONAL OFFICER AFZALPUR 145726 SHARANABASAPPA DRAWING MASTER QE-DEPARTMENT OF PUBLIC 8 17004900540008 -

Government of Karnataka Directorate of Economics and Statistics
Government of Karnataka Directorate of Economics and Statistics Modified National Agricultural Insurance Scheme - GP-wise Average Yield data for 2013-14 Experiments Average Yield District Taluk Gram Panchayath Planned Analysed (in Kgs/Hect.) Crop : JOWAR Irrigated Season RABI 1 Gulbarga 1 Afzalpur 1 Mannur 4 4 848 2 Mashal 4 4 1168 3 Balurgi 4 4 1168 4 Goura (B) 4 4 1115 5 Udachan 4 4 1230 6 Allagi (B) 4 4 1479 7 Revoor (B) 4 4 1639 8 Afzalpur (TP) 4 4 1277 9 Anoor 4 4 1707 10 Kallur 4 4 1440 11 Kognoor 4 4 1558 12 Bhairamadgi 4 4 1150 13 Athnoor 4 4 1690 14 Chowdapur 4 4 1516 15 Devalghanagapur 4 4 1135 16 Bandarwad 4 4 1188 17 Hasargundagi 4 4 852 2 Aland 18 Belamagi 4 4 2463 19 Tadkal 4 4 2248 20 Niragudi 4 4 2441 21 Jidaga 4 4 2340 22 Madanhipparaga 4 4 2511 23 Narona 4 4 2459 Page 1 of 7 Experiments Average Yield District Taluk Gram Panchayath Planned Analysed (in Kgs/Hect.) 3 Jewargi 24 Nelogi 4 4 2029 25 Kallur (K) 4 4 2095 26 Hipparga (SN) 4 4 2121 27 Kuknoor 4 4 1841 28 Yadrami 4 4 2011 29 Malli 4 4 1867 30 Wadgera 4 4 1814 31 Kuralgera 4 4 1887 32 Magangera 4 4 1898 33 Aralagundagi 4 4 1889 34 Kadakol 4 4 1880 35 Mandewal 4 4 2345 36 Jeratagi 4 4 2095 37 Ankalaga 4 4 2235 38 Itaga 4 4 2406 Page 2 of 7 Experiments Average Yield District Taluk Gram Panchayath Planned Analysed (in Kgs/Hect.) Crop : WHEAT Irrigated Season RABI 1 Gulbarga 1 Afzalpur 39 Mannur 4 4 1664 40 Mashal 4 4 1578 41 Balurgi 4 4 1453 42 Goura (B) 4 4 1837 43 Karajagi 4 4 1806 44 Udachan 4 4 1500 45 Allagi (B) 4 4 2307 46 Badadal 4 4 2401 47 Revoor (B) 4 4 1864 -

Commerce Abstract
Research Paper Volume : 4 | Issue : 2 | February 2015 • ISSN No 2277 - 8179 COMMERCE COST BENEFIT ANALYSIS OF SUGAR INDUSTRY IN KEYWORDS : Production,Cost,material, GULBARGA DISTRCIT Overhead,Profit. ASHOKKUMAR G.POLICE Head of the Department of Commerce H.K.E Siciety’s SMT V.G.College for women, Aiwan- PATIL E-Shahi,Gulbarga DR.GIRAM S.J HOD and Research Guide Vaidhynath College,Parli(V) Dist: BHEED ABSTRACT The Indian sugar industry as green industry its future determines the livelihood of millions of farmers. The growth of these companies plays a prominent role in the economic development of the nation. The growth of an industry is based on its success and productivity. It is the primary test of the success of an industry. The consumers and the government are directly or indirectly involved in this industry. If the productivity is more, there will be the technological innovations and the economic will be growth high. The productivity and efficiency mainly depend upon the age and region of the industry. INTRODUCTION To study the costing structure of sugar industry in Gulbarga dis- Sugar Industry is the second largest agro based industry in In- trict. dia, next to textile. This is the only industry, located in the ru- ral part of the country. About five crores of sugarcane farmers, To offer suitable suggestions on the basis of the findings through their dependants and a large mass of labourers are involved in the study. sugarcane cultivation, harvesting and other related ancillary activities. This constitutes 7.5 per cent of the rural population. HYPOTHESES Besides, over a five lakhs of skilled and semi-skilled workers, The researcher has framed the following hypotheses and tested mostly from the rural areas, are employed in the sugar industry, in the study. -

Curriculum Vitae
Somaiya Vidyavihar University K. J. Somaiya College of Engineering, Mumbai -77 (Constituent Academic Unit of Somaiya Vidyavihar University) Department of Mechanical Engineering CURRICULUM VITAE Part I: PERSONAL INFORMATION Name Dr. Siddappa Sharanappa Bhusnoor Date of Birth June 1, 1972 Language Known English, Hindi, Kannada, Marathi Present Post Professor , Department of Mechanical Engineering K. J. Somaiya College of Engineering, Mumbai- 400 077 Address for K. J. Somaiya College of Engineering, Correspondence Mechanical Engineering Department Vidyanagar, Vidyavihar, Mumbai- 400 077 Email: [email protected]; [email protected] Mobile: 99 20 53 45 14 / 91 67 26 45 38 Phone: 022- 66 44 95 03 / 66 44 94 75 Academic Record starting with graduation Degree University/Board /Institute Year of % of marks Passing /CGPA B.E. Gulbarga University, Karnataka, India, Aug, 1995 76.46 (Mechanical) (P.D.A. College of Engineering, Gulbarga) M.Tech. Indian Institute of Technology Delhi, May, 2006 8.846 (Energy Studies) Hauz Khas, New Delhi Ph. D. Indian Institute of Technology Bombay, Feb, 2016 Awarded Powai, Mumbai Thesis Details Ph. D. Modelling and Experimental Studies for Particle Transport by Thermophoresis. (IIT Bombay) M. Tech. Production and Utilization of Biodiesel (Linseed Oil Methyl Ester) in a Compression Ignition Engines. (IIT Delhi) 1 Somaiya Vidyavihar University K. J. Somaiya College of Engineering, Mumbai -77 (Constituent Academic Unit of Somaiya Vidyavihar University) Department of Mechanical Engineering Professional Experience Employer Position held From To Professor (CONCOL/ICC/04 of July 2016 Till date 2012 and CONCOL/09/2015-16, December 14, 2015) Professor and Head of the July 2016 Aug 12, 2018 Department (CONCOL/ICC/04 of 2012 and K. -
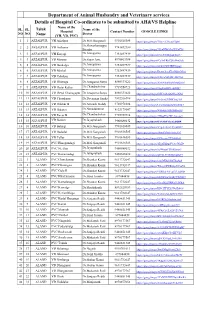
Department of Animal Husbandry and Veterinary Services Details of Hospital Co-Ordinates to Be Submitted to AH&VS Helpline Name of the SL
Department of Animal Husbandry and Veterinary services Details of Hospital Co-ordinates to be submitted to AH&VS Helpline Name of the SL. SL. Taluk Name of the Hospital Contact Number GOOGLE LINKS NO NO Doctor Name (VH, VD, PVC) 1 1 AFZALPUR VH Afzalpur Dr.M.S.Ganganali 9901616585 https://goo.gl/maps/7JJbcvT3AcjoC5a86 Dr.Shivasharnappa 2 2 AFZALPUR VH Anthanor 9741692180 Biradar https://goo.gl/maps/7GenBBbALcCFUnJY8 Dr.Annapurna 3 3 AFZALPUR VH Karjagi 7353497939 https://goo.gl/maps/p7nvP8HBgLpDrap37 Avate 4 4 AFZALPUR VD Mannur Dr.Sagar Avte 8970863988 https://goo.gl/maps/rCy1sYRkCZhbMm5aA Dr.Annapurna 5 5 AFZALPUR VD Bankalga 7353497939 https://goo.gl/maps/arndwZdWJRRfygog8 Avate Dr.Annapurna 6 6 AFZALPUR VD Mashal 7353497939 https://goo.gl/maps/Fumw3hwkXwDQyxMA8 Avate Dr.Annapurna 7 7 AFZALPUR VD Udachan 7353497939 https://goo.gl/maps/sCHvwSSUR1vMir9G6 Avate 8 8 AFZALPUR VD Ghatraga Dr.Anupama Surya 8880333622 https://goo.gl/maps/XMrNskuWkMMpQbfu9 Dr.Chandrashekar 9 9 AFZALPUR VD Desai Kallur 9739550723 https://goo.gl/maps/zAg5S6HZzerju5zH9 Yellagouda 10 10 AFZALPUR VD Deval Ghanagapur Dr.Anupama Surya 8880333622 https://goo.gl/maps/rMV2o2ktQQSbcaNA6 11 11 AFZALPUR VD Choudapur Dr.Devanaga Sanka 7892266098 https://goo.gl/maps/Nu155j2Gf8MYuL9x9 12 12 AFZALPUR VD Gobbur B Dr.Avinash Reddy 9720978384 https://goo.gl/maps/UmoH6XZz1aWro58K7 Dr.Nanadakumar 13 13 AFZALPUR VD Bidanor 8123179007 https://goo.gl/maps/WEkhj6cMB9BtBm5i8 Mathapathi Dr.Chandrashekar 14 14 AFZALPUR VD Revur B 7795959714 https://goo.gl/maps/25KqZYg7RPe3wrow9 Kenganal -

Dist Name Taluk Name GP Name New Accoun No Bank Name Branch Name IFSC Code Rel Amount Gulbarga Afzalpur Kallur 1559 Krishna Gram
Dist_name taluk_name GP_name New Accoun no Bank_name Branch name IFSC code rel_amount Gulbarga Afzalpur Kallur 1559 Krishna Grameena bank (KGB) Kallur 15.00 Gulbarga Afzalpur Ballurgi 30833298777 State Bank of India (SBI) Afzalpur SBIN0011581 10.00 Afzalpur Total 25.00 Gulbarga Aland Darga Sirur 11180276742 State Bank of India (SBI) Madanhipparga SBIN0005981 5.00 Gulbarga Aland Madan Hipparga 11180276731 State Bank of India (SBI) Madanhipparga SBIN0005981 5.00 Gulbarga Aland Koralli 4513 Krishna Grameena bank (KGB) Bhusnoor 5.00 Gulbarga Aland Khajuri 5034 Krishna Grameena bank (KGB) Khajuri 5.00 Gulbarga Aland Kavalga 4531 Krishna Grameena bank (KGB) Bhusnoor 4.00 Gulbarga Aland Kamalanagar 5192 Krishna Grameena bank (KGB) V.K.Salgar 5.00 Gulbarga Aland Yalsangi 5086 Krishna Grameena bank (KGB) Madiyal 5.00 Gulbarga Aland Kadaganchi 10814186702 State Bank of India (SBI) Kadaganchi SBIN0003825 5.00 Gulbarga Aland Alanga 5038 Krishna Grameena bank (KGB) Khajuri 5.00 Gulbarga Aland Ambalga 3480 Krishna Grameena bank (KGB) Ladamugali 5.00 Gulbarga Aland Jidaga 17230 Krishna Grameena bank (KGB) Aland 5.00 Gulbarga Aland Hodloor 5044 Krishna Grameena bank (KGB) Khajuri 4.00 Gulbarga Aland Belamagi 5209 Krishna Grameena bank (KGB) V.K.Salgar 3.00 Gulbarga Aland Chnchansoor 3217 Krishna Grameena bank (KGB) Chinchansoor 5.00 Gulbarga Aland Bhusnur 4540 Krishna Grameena bank (KGB) Bhusnoor 4.00 Gulbarga Aland Bhodhan 201 Krishna Grameena bank (KGB) Narona 4.00 Gulbarga Aland Dhangapur 11132157326 State Bank of India (SBI) Nimbarga SBIN0005981 -

Government AYUSHMAN BHARAT
AYUSHMAN BHARAT - AROGYA KARNATAKA EMPANELLED HOSPITALS LIST Govt/Priv Sl.no Hospital Name Address District Taluk Division Contact Mail id Scheme Speciality ate Government Community Health Centre Obstetrics and VijayapuraDevanahalli Ayushman gynaecology Community Health Centre Road Vijayapura Bangalore chcvijayapura@g 1 Bangalore Devanahalli govt 8027668505 Bharat - Arogya Dental Vijayapura Devanhalli division mail.com Karnataka Simple secondary general TalukBengaluru Rural- procedure 562135 Obstetrics and Ayushman Community Health Centre B M Road Kengeri Kote Bangalore girijagowdab@g gynaecology Paediatrics 2 Bangalore Bengaluru govt 8028483265 Bharat - Arogya Kengeri Bangalore 560060 division mail.com Simple secondary General Karnataka procedure Paediatric surgeries Community Health Centre Obstetrics and Ayushman Community Health Centre ThyamagondluNear Police Bangalore thyamagondluchc gynaecology 3 Bangalore Nelemangala govt 8027731202 Bharat - Arogya Thyamagondlu StationBangalore - Rural- division @gmail.com Dental Karnataka 562132 Simple secondary General procedure Paediatric Surgery Community Health Center Ayushman General Medicine Community Health Center Near Water Bangalore mophcavalhalli@ 4 Bangalore Bengaluru govt 8028473108 Bharat - Arogya Dental Avalahalli PlantationBangalore - division gmail.com Karnataka Obstetrics and Urban-560049 gynaecology Dental Obstetrics and Ayushman Tavarekere Hobli South Bangalore dr.candrappacercl gynaecology 5 CHC Chandrappa Cercle Bangalore Bengaluru govt 8028438330 Bharat - Arogya TalukBengaluru -

Name of the Court V.Addl. District & Sessions Judge
Name of the Court V.Addl. District & Sessions Judge Dharwad sitting at Hubballi. List of cases as per the SOP guidelines issued by the Hon'ble High Court of Karnataka's Notification dated 26-05-2020 which are posted on Date 09-06-2020. MORNING SESSION Name of the Plaintiff/claimant Name of the counsel Sl.No. Case No. Stage of case counsel for the Defendant/Respondent for respondent claimant SMT.BHIMAVVA W/O KALLAPPA GOKUL 1 RA.119/2017 Arguments Vs S.D.Hosamani R.G.Matti SMT.DYAMAVVA W/O BHIMANNA SHINGADE SMT.SIDDAVVA W/O CHENNAYYA Vs 2 RA.226/2017 Arguments SMT.SAROJAMMA W/O K.S.Patil S.K.Desai VENKATRAMAN CHITTA NIRMALA alias NEELAWWA W/O LATE HANAMANTHAGOUDA PATIL 3 RA.231/2017 Arguments Vs P.S.Udikeri A.G.Rehamansha BASANAGOUDA VEERANAGOUDA PATIL SMT.MODINBI W/O KHADARSAB KUNDGOL 4 RA.282/2017 Arguments Vs P.S.Naregal R.G.Hosagoudar SMT.AMMAJAN W/O KUTUBUDDIN CHENGAPURI VIJAYA W/O ISHWARAPPA KAMMAR Vs 5 RA.286/2017 Arguments SMT.PARVATEVVA W/O R.B.Kale P.S.Naregal SOMARAYAPPA BADIGER SMT.MEHABOOBBI W/O SALIM SHAIKH 6 RA.287/2017 Arguments Vs A.G.Walasang C.H.Sajjanshettar MEHABOOBASAB S/O IMSALISAB MULLA APPASAHEB S/O BABUSAHEB KATTIMANI 7 MA.36/2017 Arguments Vs S.V.Dhage S.M.Sheelawant THE COMMISSIONER ,HDMC, HUBBALLI FAKKIRAPPA S/O BASAPPA ANGADI Vs 8 RA.313/2017 Arguments SMT.VIJAYALAXMI W/O SIDDAPPA C.G.Pattar N.K.Kayakamath MALLANNAVAR MAHAVEER S/O NEMARAJAPPA SOOJI Vs 9 RA.321/2017 Arguments SMT.NINGAWWA KOM YALLAPPA S.C.Jainar A.A.Kamanagar HULKOPPA - SINCE DECEASED DELETED AS PER ORDER DTD 20- 02-2015 SIDDAYYA S/O GURUPADAYYA HIREMATH 10 RA.05/2018 Arguments Vs B.M.Kulageri P.A.Shettar SMT.PARVATEWWA W/O GANGAYYA HIREMATH AFTER NOON SESSION SHIVAPPA S/O. -

Government of Karnataka Directorate of Economics and Statistics
Government of Karnataka Directorate of Economics and Statistics Modified National Agricultural Insurance Scheme - GP-wise Average Yield data for 2013-14 Experiments Average Yield District Taluk Gram Panchayath Planned Analysed (in Kgs/Hect.) Crop : RICE Irrigated Season KHARIF 1 Gulbarga 1 Chittapur 1 Satnoor 4 4 2073 2 Sannathi 4 4 2738 2 Gulbarga 3 Farthabad 4 2 2505 3 Jewargi 4 Ganwar 4 4 1829 5 Billawar 4 4 3042 6 Ballabatti 4 4 2455 7 Malli 4 4 2583 8 Wadgera 4 4 2062 9 Kuralgera 4 4 2300 10 Magangera 4 4 2715 11 Aralagundagi 4 4 2061 4 Sedam 12 Lingampalli 4 4 3170 13 Itkal 4 4 3544 14 Kanagadda 4 4 3588 15 Motakpalli 4 4 3679 16 Ribbanpalli 4 4 3588 17 Kolkunda 4 4 3308 Page 1 of 3 Experiments Average Yield District Taluk Gram Panchayath Planned Analysed (in Kgs/Hect.) Crop : MAIZE Irrigated Season KHARIF 1 Gulbarga 1 Afzalpur 18 Goura (B) 4 4 2421 19 Karajagi 4 4 2167 20 Udachan 4 4 2317 21 Revoor (B) 4 4 4245 22 Afzalpur (TP) 4 4 3481 23 Kallur 4 4 3819 2 Gulbarga 24 Kalmud 4 4 4562 25 Nandikur 4 4 3190 3 Jewargi 26 Kuralgera 4 4 2629 27 Aralagundagi 4 4 3066 Crop : TUR Irrigated Season KHARIF 1 Gulbarga 1 Afzalpur 28 Mannur 4 4 1199 29 Karajagi 4 4 1557 30 Udachan 4 4 1347 31 Allagi (B) 4 4 1682 32 Anoor 4 4 1480 33 Kallur 4 4 1668 34 Athnoor 4 4 1588 35 Chowdapur 4 4 1967 36 Devalghanagapur 4 4 1773 37 Gobbur (B) 4 4 1777 38 Bandarwad 4 4 1889 39 Hasargundagi 4 4 1777 2 Gulbarga 40 Kurikota 4 4 1473 Page 2 of 3 Experiments Average Yield District Taluk Gram Panchayath Planned Analysed (in Kgs/Hect.) 3 Jewargi 41 Nelogi 4 4 1292