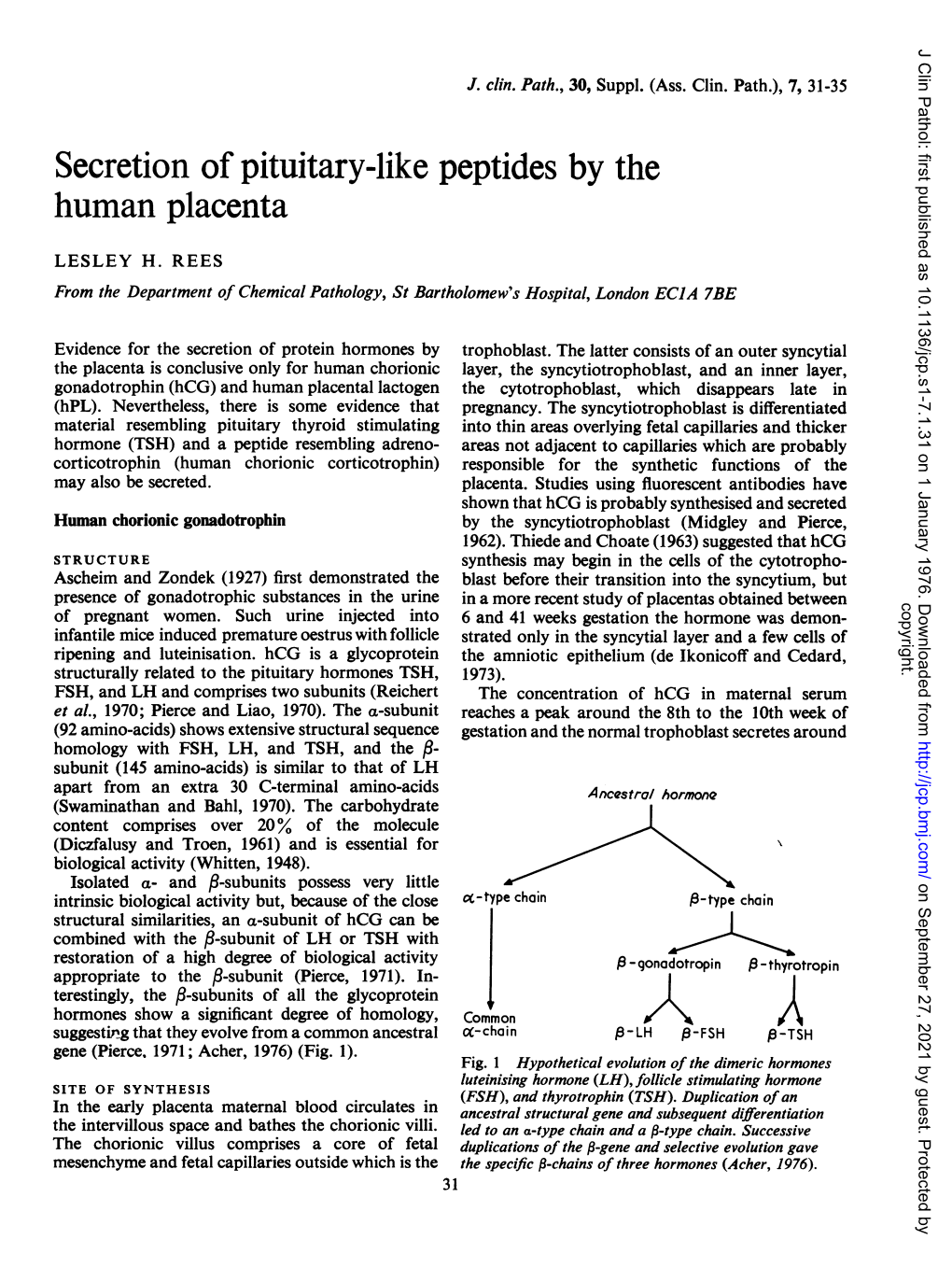
Secretion of Pituitary-Like Peptides by the Human Placenta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Leptin Stimulates Pituitary Prolactin Release Through an Extracellular Signal-Regulated Kinase-Dependent Pathway
275 Leptin stimulates pituitary prolactin release through an extracellular signal-regulated kinase-dependent pathway Christian K Tipsmark, Christina N Strom, Sean T Bailey and Russell J Borski Department of Zoology, North Carolina State University, Raleigh, North Carolina 27695-7617, USA (Correspondence should be addressed to C K Tipsmark who is now at Institute of Biology, University of Southern Denmark, Campusvej 55, DK-5230 Odense M, Denmark; Email: [email protected]) Abstract Leptin was initially identified as a regulator of appetite and leptin are mediated by the activation of extracellular signal- weight control centers in the hypothalamus, but appears to be regulated kinase (ERK1/2) but nothing is known about the involved in a number of physiological processes. This study cellular mechanisms by which leptin might regulate PRL was carried out to examine the possible role of leptin in secretion in vertebrates. We therefore tested whether regulating prolactin (PRL) release using the teleost pituitary ERK1/2 might be involved in the leptin PRL response and model system. This advantageous system allows isolation of a found that the ERK inhibitor, PD98059, hindered leptin- nearly pure population of lactotropes in their natural, in situ induced PRL release. We further analyzed leptin response by aggregated state. The rostral pars distalis were dissected from quantifying tyrosine and threonine phosphorylation of tilapia pituitaries and exposed to varying concentrations of ERK1/2 using western blots. One hour incubation with leptin (0, 1, 10, 100 nM) for 1 h. Release of PRL was leptin induced a concentration-dependent increase in stimulated by leptin in a potent and concentration-dependent phosphorylated, and thus active, ERK1/2. -

Rfamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential
Pharmaceuticals 2011, 4, 1248-1280; doi:10.3390/ph4091248 OPEN ACCESS Pharmaceuticals ISSN 1424-8247 www.mdpi.com/journal/pharmaceuticals Review RFamide Peptides: Structure, Function, Mechanisms and Pharmaceutical Potential Maria Findeisen †, Daniel Rathmann † and Annette G. Beck-Sickinger * Institute of Biochemistry, Leipzig University, Brüderstraße 34, 04103 Leipzig, Germany; E-Mails: [email protected] (M.F.); [email protected] (D.R.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-341-9736900; Fax: +49-341-9736909. Received: 29 August 2011; in revised form: 9 September 2011 / Accepted: 15 September 2011 / Published: 21 September 2011 Abstract: Different neuropeptides, all containing a common carboxy-terminal RFamide sequence, have been characterized as ligands of the RFamide peptide receptor family. Currently, five subgroups have been characterized with respect to their N-terminal sequence and hence cover a wide pattern of biological functions, like important neuroendocrine, behavioral, sensory and automatic functions. The RFamide peptide receptor family represents a multiligand/multireceptor system, as many ligands are recognized by several GPCR subtypes within one family. Multireceptor systems are often susceptible to cross-reactions, as their numerous ligands are frequently closely related. In this review we focus on recent results in the field of structure-activity studies as well as mutational exploration of crucial positions within this GPCR system. The review summarizes the reported peptide analogs and recently developed small molecule ligands (agonists and antagonists) to highlight the current understanding of the pharmacophoric elements, required for affinity and activity at the receptor family. -

Co-Regulation of Hormone Receptors, Neuropeptides, and Steroidogenic Enzymes 2 Across the Vertebrate Social Behavior Network 3 4 Brent M
bioRxiv preprint doi: https://doi.org/10.1101/435024; this version posted October 4, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Co-regulation of hormone receptors, neuropeptides, and steroidogenic enzymes 2 across the vertebrate social behavior network 3 4 Brent M. Horton1, T. Brandt Ryder2, Ignacio T. Moore3, Christopher N. 5 Balakrishnan4,* 6 1Millersville University, Department of Biology 7 2Smithsonian Conservation Biology Institute, Migratory Bird Center 8 3Virginia Tech, Department of Biological Sciences 9 4East Carolina University, Department of Biology 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 1 bioRxiv preprint doi: https://doi.org/10.1101/435024; this version posted October 4, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Running Title: Gene expression in the social behavior network 2 Keywords: dominance, systems biology, songbird, territoriality, genome 3 Corresponding Author: 4 Christopher Balakrishnan 5 East Carolina University 6 Department of Biology 7 Howell Science Complex 8 Greenville, NC, USA 27858 9 [email protected] 10 2 bioRxiv preprint doi: https://doi.org/10.1101/435024; this version posted October 4, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Lactation and Appetite-Regulating Hormones: Increased Maternal Plasma Peptide YY Concentrations 3–6 Months Postpartum
Downloaded from British Journal of Nutrition (2015), 114, 1203–1208 doi:10.1017/S0007114515002536 © The Authors 2015 https://www.cambridge.org/core Lactation and appetite-regulating hormones: increased maternal plasma peptide YY concentrations 3–6 months postpartum Greisa Vila1,*, Judith Hopfgartner1, Gabriele Grimm2, Sabina M. Baumgartner-Parzer1, . IP address: Alexandra Kautzky-Willer1, Martin Clodi1 and Anton Luger1 1Department of Internal Medicine III, Division of Endocrinology and Metabolism, Medical University of Vienna, 170.106.34.90 Vienna 1090, Austria 2Department of Medical and Chemical Laboratory Diagnostics, Medical University of Vienna, Vienna 1090, Austria – – – (Submitted 12 October 2014 Final revision received 27 May 2015 Accepted 11 June 2015 First published online 24 August 2015) , on 28 Sep 2021 at 07:22:14 Abstract Breast-feeding is associated with maternal hormonal and metabolic changes ensuring adequate milk production. In this study, we investigate the impact of breast-feeding on the profile of changes in maternal appetite-regulating hormones 3–6 months postpartum. Study participants were age- and BMI-matched lactating mothers (n 10), non-lactating mothers (n 9) and women without any history of pregnancy or breast-feeding in the previous 12 months (control group, n 10). During study sessions, young mothers breast-fed or bottle-fed their babies, and maternal blood , subject to the Cambridge Core terms of use, available at samples were collected at five time points during 90 min: before, during and after feeding the babies. Outcome parameters were plasma concentrations of ghrelin, peptide YY (PYY), leptin, adiponectin, prolactin, cortisol, insulin, glucose and lipid values. At baseline, circulating PYY concentrations were significantly increased in lactating mothers (100·3(SE 6·7) pg/ml) v. -

The Effect of Very-Low-Calorie Diet on Mrna Expression of Inflammation
JCEM ONLINE Hot Topics in Translational Endocrinology—Endocrine Research The Effect of Very-Low-Calorie Diet on mRNA Expression of Inflammation-Related Genes in Subcutaneous Adipose Tissue and Peripheral Monocytes of Obese Patients with Type 2 Diabetes Mellitus Downloaded from https://academic.oup.com/jcem/article/96/4/E606/2720876 by guest on 26 September 2021 M. Mraz, Z. Lacinova, J. Drapalova, D. Haluzikova, A. Horinek, M. Matoulek, P. Trachta, P. Kavalkova, S. Svacina, and M. Haluzik Third Department of Medicine (M.Mr., Z.L., J.D., D.H., A.H., M.Ma., P.T., P.K., S.S., M.H.) and Department of Sports Medicine (D.H.), General University Hospital and First Medical Faculty, Charles University, 128 08 Prague 2, Czech Republic Context: Low-grade inflammation links obesity, type 2 diabetes mellitus (T2DM), and cardiovas- cular diseases. Objective: To explore the expression profile of genes involved in inflammatory pathways in adipose tissue and peripheral monocytes (PM) of obese patients with and without T2DM at baseline and after dietary intervention. Design: Two-week intervention study with very-low-calorie diet (VLCD). Setting: University hospital. Patients: Twelve obese females with T2DM, 8 obese nondiabetic females (OB) and 15 healthy age-matched females. Intervention: Two weeks of VLCD (2500 kJ/d). Main Outcome Measures: Metabolic parameters, circulating cytokines, hormones, and mRNA expression of 39 genes in sc adipose tissue (SCAT) and PM. Results: Both T2DM and OB group had significantly increased serum concentrations of circulating proinflammatory factors (C-reactive protein, TNF␣, IL-6, IL-8), mRNA expression of macrophage antigen CD68 and proinflammatory chemokines (CCL-2, -3, -7, -8, -17, -22) in SCAT and comple- mentary chemokine receptors (CCR-1, -2, -3, -5) and other proinflammatory receptors (toll-like receptor 2 and 4, TNF receptor superfamily 1A and 1B, IL-6R) in PM, with OB group showing less pronounced chemoattracting and proinflammatory profile compared to T2DM group. -

Views of the NIDA, NINDS Or the National Summed Across the Three Auditory Forebrain Lobule Sec- Institutes of Health
Xie et al. BMC Biology 2010, 8:28 http://www.biomedcentral.com/1741-7007/8/28 RESEARCH ARTICLE Open Access The zebra finch neuropeptidome: prediction, detection and expression Fang Xie1, Sarah E London2,6, Bruce R Southey1,3, Suresh P Annangudi1,6, Andinet Amare1, Sandra L Rodriguez-Zas2,3,5, David F Clayton2,4,5,6, Jonathan V Sweedler1,2,5,6* Abstract Background: Among songbirds, the zebra finch (Taeniopygia guttata) is an excellent model system for investigating the neural mechanisms underlying complex behaviours such as vocal communication, learning and social interactions. Neuropeptides and peptide hormones are cell-to-cell signalling molecules known to mediate similar behaviours in other animals. However, in the zebra finch, this information is limited. With the newly-released zebra finch genome as a foundation, we combined bioinformatics, mass-spectrometry (MS)-enabled peptidomics and molecular techniques to identify the complete suite of neuropeptide prohormones and final peptide products and their distributions. Results: Complementary bioinformatic resources were integrated to survey the zebra finch genome, identifying 70 putative prohormones. Ninety peptides derived from 24 predicted prohormones were characterized using several MS platforms; tandem MS confirmed a majority of the sequences. Most of the peptides described here were not known in the zebra finch or other avian species, although homologous prohormones exist in the chicken genome. Among the zebra finch peptides discovered were several unique vasoactive intestinal and adenylate cyclase activating polypeptide 1 peptides created by cleavage at sites previously unreported in mammalian prohormones. MS-based profiling of brain areas required for singing detected 13 peptides within one brain nucleus, HVC; in situ hybridization detected 13 of the 15 prohormone genes examined within at least one major song control nucleus. -

The Hypothalamo-Prolactin Axis 226:2 T101–T122 Thematic Review
D R Grattan The hypothalamo-prolactin axis 226:2 T101–T122 Thematic Review Open Access 60 YEARS OF NEUROENDOCRINOLOGY The hypothalamo-prolactin axis David R Grattan1,2 Correspondence should be addressed 1Centre for Neuroendocrinology and Department of Anatomy, University of Otago, to D R Grattan PO Box 913, Dunedin 9054, New Zealand Email 2Maurice Wilkins Centre for Molecular Biodiscovery, Auckland, New Zealand [email protected] Abstract The hypothalamic control of prolactin secretion is different from other anterior pituitary Key Words hormones, in that it is predominantly inhibitory, by means of dopamine from the tubero- " prolactin infundibular dopamine neurons. In addition, prolactin does not have an endocrine target " tuberoinfundibular tissue, and therefore lacks the classical feedback pathway to regulate its secretion. Instead, dopamine neurons it is regulated by short loop feedback, whereby prolactin itself acts in the brain to stimulate " pregnancy production of dopamine and thereby inhibit its own secretion. Finally, despite its relatively " lactation simplename, prolactin has a broad range offunctions in the body,in addition to its defining role " prolactin-releasing factor in promoting lactation. As such, the hypothalamo-prolactin axis has many characteristics that are quite distinct from other hypothalamo-pituitary systems. This review will provide a brief overview of our current understanding of the neuroendocrine control of prolactin secretion, in particular focusing on the plasticity evident in this system, which keeps prolactin secretion at low levels most of the time, but enables extended periods of hyperprolactinemia when Journal of Endocrinology necessary for lactation. Key prolactin functions beyond milk production will be discussed, particularly focusing on the role of prolactin in inducing adaptive responses in multiple different systems to facilitate lactation, and the consequences if prolactin action is impaired. -

Peptide YY (PYY)3–36 Modulates Thyrotropin Secretion in Rats
459 Peptide YY (PYY)3–36 modulates thyrotropin secretion in rats K J Oliveira, G S M Paula, R H Costa-e-Sousa, L L Souza, D C Moraes, F H Curty and C C Pazos-Moura Laborato´rio de Endocrinologia Molecular, Instituto de Biofı´sica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro 21949-900, Brazil (Requests for offprints should be addressed to C C Pazos-Moura; Email: [email protected]) Abstract Peptide YY (PYY)3-36 is a gut-derived hormone, with a However, in fasted rats, PYY3-36 at both doses elicited a proposed role in central mediation of postprandial satiety signals, significant rise (approximately twofold increase, P!0.05) in as well as in long-term energy balance. In addition, recently,the serum TSH observed 15 min after the hormone injection. ability of the hormone to regulate gonadotropin secretion, PYY3-36 treatment did not modify significantly serum T4,T3,or acting at pituitary and at hypothalamus has been reported. Here, leptin. Therefore, in the present paper, we have demonstrated we examined PYY3-36 effects on thyrotropin (TSH) secretion, that the gut hormone PYY3-36 acts directly on the pituitary both in vitro and in vivo.PYY3-36-incubated rat pituitary glands gland to inhibit TSH release, and in the fasting situation, in vivo, showed a dose-dependent decrease in TSH release, with 44 and when serum PYY3-36 is reduced, the activity of thyroid axis is K K 62% reduction at 10 8 and 10 6 M(P!0.05 and P!0.001 reduced as well. -

Placental Lactogen-I (PL-I) Target Tissues Identified with an Alkaline Phosphatase–PL-I Fusion Protein
Volume 46(6): 737–743, 1998 The Journal of Histochemistry & Cytochemistry http://www.jhc.org ARTICLE Placental Lactogen-I (PL-I) Target Tissues Identified with an Alkaline Phosphatase–PL-I Fusion Protein Heiner Müller,1 Guoli Dai, and Michael J. Soares Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, Kansas SUMMARY The rat placenta expresses a family of genes related to prolactin (PRL). Target tissues and physiological roles for many members of the PRL family have yet to be deter- mined. In this investigation we evaluated the use of an alkaline phosphatase (AP) tag for monitoring the behavior of a prototypical member of the PRL family, placental lactogen-I (PL-I). A probe was generated consisting of a fusion protein of human placental AP and rat KEY WORDS PL-I (AP–PL-I). The AP–PL-I construct was stably expressed in 293 human fetal kidney cells, as alkaline phosphatase fusion was the unmodified AP vector that served as a control. AP activity was monitored with a protein colorimetric assay in conditioned medium from transfected cells. Immunoreactivity and ovary, corpus luteum prolactin PRL-like biological activities of the AP–PL-I fusion protein were demonstrated by immuno- receptors blotting and the Nb2 lymphoma cell proliferation assay, respectively. AP–PL-I specifically liver, hepatic prolactin bound to tissue sections known to express the PRL receptor, including the ovary, liver, and receptors choroid plexus. Binding of AP–PL-I to tissues was specific and could be competed with ovine Nb2 lymphoma cells PRL. The results indicate that AP is an effective tag for monitoring the behavior of PL-I and placental lactogen-I suggest that this labeling system may also be useful for monitoring the actions of other pregnancy members of the PRL family. -

Product Datasheet NPVF Antibody GP-1080-50
Product Datasheet NPVF Antibody GP-1080-50 Unit Size: 0.05 ml Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles. Protocols, Publications, Related Products, Reviews, Research Tools and Images at: www.novusbio.com/GP-1080-50 Updated 2/5/2017 v.20.1 Earn rewards for product reviews and publications. Submit a publication at www.novusbio.com/publications Submit a review at www.novusbio.com/reviews/destination/GP-1080-50 Page 1 of 3 v.20.1 Updated 2/5/2017 GP-1080-50 NPVF Antibody Product Information Unit Size 0.05 ml Concentration This product is unpurified. The exact concentration of antibody is not quantifiable. Storage Store at 4C short term. Aliquot and store at -20C long term. Avoid freeze-thaw cycles. Clonality Polyclonal Preservative No Preservative Reconstitution Instructions Reconstitute with 50 ul sterilized water. Centrifuge to remove any insoluble material. Purity Unpurified Buffer Whole antisera Product Description Host Guinea Pig Gene ID 64111 Gene Symbol NPVF Species Sheep Reactivity Notes Sheep Specificity/Sensitivity Specificity was demonstrated by immunohistochemistry. Immunogen A synthetic peptide (VPNLPQRF) corresponding to the amino acids 124-131 from human Neuropeptide VF. Neuropeptide VF is the precursor of the neuropeptides NPSF (RFRP-1), RFRP-2 and RFRP-3. The synthetic peptide was conjugated to a carrier protein KLH to enhance the immunological response. Notes Reconstitute in 50 µl sterile water. Centrifuge to remove any insoluble material. Product Application Details Applications Immunohistochemistry, Immunohistochemistry-Paraffin Recommended Dilutions Immunohistochemistry, Immunohistochemistry-Paraffin 1:2000 Application Notes IHC. A concentration of 1 in 2000 is recommended for IHC. -

Preliminary Experience with Resistin Assessment in Common Population
Biomed. Papers 146(2), 47–49 (2002) 47 © D. Stejskal, J. Prošková, S. Adamovská, R. Juráková, J. Bartek PRELIMINARY EXPERIENCE WITH RESISTIN ASSESSMENT IN COMMON POPULATION David Stejskala, Jitka Proškováa, Sylva Adamovskáa, Renata Jurákováa, Josef Bartekb a Department of Laboratory Medicine, Hospital Šternberk b Institute of Medical Chemistry and Biochemistry, Medical Faculty, Hněvotínská 3, 775 15 Olomouc Received: September 20, 2002 Key words: Resistin / Serum concentration Resistin is a signal peptide produced by adipose tissue. Mice models have confirmed that resistin may play an important role in insulin resistance. Its function in the human organism has not been elucidated yet. Since in common population the resistin concentrations are not known (no validated commercial set is available), we performed resistin assessment using the ELISA method (with satisfying analytical characteristics) in a population of 123 non-obese probands without signs of insulin resistance and/or inflammation. Mean resistin values amounted to 14.3 ng/ml (reference limit of 7.3–21.3 ng/ml). INTRODUCTION bolism of insulin, glucose and adipocyte metabolism in general. It has been found that resistin expression in Several years ago, a new signal molecule was disco- adipocytes is influenced by certain cytokines, increased vered and termed resistin (12.5 kDa). It is a product of in hyperglycemia, hypercorticolism (administration of the RSTN gene that belongs to the category of proteins dexamethasone to mice resulted in elevated mRNA rich in cysteine (RELM family); some authors also use resistin 2.5–3.5×) and decreased it after administration the terms ADSF (Adipose Tissue-Specific Secretory of insulin, thiasolindion, TNF-alpha (up by 80%), adre- Factor) or FIZZ3 (Found in Inflammatory Zone). -

Effect of Corticotropin-Like Intermediate Lobe Peptide on Pancreatic Exocrine Function in Isolated Rat Pancreatic Lobules
Effect of corticotropin-like intermediate lobe peptide on pancreatic exocrine function in isolated rat pancreatic lobules. J B Marshall, … , L D Manning, A J McCullough J Clin Invest. 1984;74(5):1886-1889. https://doi.org/10.1172/JCI111608. Research Article Naturally occurring derivatives of pro-opiomelanocortin (POMC) have been identified in various extra-pituitary sites, including the endocrine and exocrine pancreas. Corticotropin-like intermediate lobe peptide (CLIP = ACTH18-39), a naturally occurring derivative of POMC, has been suggested to be an insulin secretagogue. To determine whether CLIP might also affect the exocrine pancreas, we measured its effect on amylase secretion and protein synthesis and secretion in isolated rat pancreatic lobules. Lobules were dual-pulsed with trace amounts of 14C- and 3H-leucine, both in the presence and absence of CLIP (10(-9)-10(-6) M), using a technique that permitted the labeling of both the synthetic and secretory compartments. The effect of CLIP on protein synthesis was determined by comparing 3H-leucine incorporation into lobules with and without CLIP. The secretory effect of CLIP was determined by measuring (a) secreted 14C-labeled protein as a percent of total incorporated radiolabeled protein, and (b) amylase release into incubation medium. The effect of CLIP on amylase release was compared with that of secretin, cholecystokinin-octapeptide, and carbamylcholine. To localize the biologically active region of CLIP, we similarly studied synthetic ACTH25-39. We demonstrated that CLIP stimulates amylase and protein secretion in a dose-dependent manner and is of similar potency to secretin and carbamylcholine. This effect appears to require the ACTH18-24 region of CLIP and results from stimulus-secretion coupling rather than augmented protein synthesis.