US Army Photography Course Laboratory Procedures SS0509
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Still Photography
Still Photography Soumik Mitra, Published by - Jharkhand Rai University Subject: STILL PHOTOGRAPHY Credits: 4 SYLLABUS Introduction to Photography Beginning of Photography; People who shaped up Photography. Camera; Lenses & Accessories - I What a Camera; Types of Camera; TLR; APS & Digital Cameras; Single-Lens Reflex Cameras. Camera; Lenses & Accessories - II Photographic Lenses; Using Different Lenses; Filters. Exposure & Light Understanding Exposure; Exposure in Practical Use. Photogram Introduction; Making Photogram. Darkroom Practice Introduction to Basic Printing; Photographic Papers; Chemicals for Printing. Suggested Readings: 1. Still Photography: the Problematic Model, Lew Thomas, Peter D'Agostino, NFS Press. 2. Images of Information: Still Photography in the Social Sciences, Jon Wagner, 3. Photographic Tools for Teachers: Still Photography, Roy A. Frye. Introduction to Photography STILL PHOTOGRAPHY Course Descriptions The department of Photography at the IFT offers a provocative and experimental curriculum in the setting of a large, diversified university. As one of the pioneers programs of graduate and undergraduate study in photography in the India , we aim at providing the best to our students to help them relate practical studies in art & craft in professional context. The Photography program combines the teaching of craft, history, and contemporary ideas with the critical examination of conventional forms of art making. The curriculum at IFT is designed to give students the technical training and aesthetic awareness to develop a strong individual expression as an artist. The faculty represents a broad range of interests and aesthetics, with course offerings often reflecting their individual passions and concerns. In this fundamental course, students will identify basic photographic tools and their intended purposes, including the proper use of various camera systems, light meters and film selection. -

PRODUCT CATALOGUE All Prices Are Inclusive of VAT HOW to ORDER
ISSUE 29 PRODUCT CATALOGUE All prices are inclusive of VAT HOW TO ORDER TELEPHONE - 01789 739200 With your credit card or debit card ready between 8.30am - 5.30pm Monday to Friday. Outside these hours your order will be handled by answerphone. We check our answerphone first thing every weekday morning, so this will not delay your order. WEB Providing the items you order are in stock and the order is received before 2pm, we aim to deliver to you the next WEBSITE: www.theimagingwarehouse.com working day*. Visit our totally secure website for online ordering of the thousands of products we have in stock ready for immedi- FAX - 01789 731569 ate despatch. Check out our latest offers whilst there! Fax your completed order form with your debit or credit card details to this number. Providing the items are in stock and your With over 2,500 products available from stock, a order is faxed before 2pm, we aim to deliver to you the next range of quality used equipment and a team of working day*. technical staff boasting over 75 years of knowl- edge and experience, we are a one-stop shop for all your darkroom needs. Major brands including: POST Ilford, Kodak, Agfa, Fuji, Fotospeed, Rollei, Adox, FREEPOST RSHZ-JGBS-LGKJ (+ standard address) Kentmere, Harman and more. We continue to be Post your completed order form with your cheque, postal order, the world-leading manufacturer of slot-processors and archival debit or credit card details in an envelope using the Freepost washers, offering a full selection of space saving and environ- address. -

UK Photography Activity Badge
making a start in photography Jessops is proud to support The Scout Association and sponsor the Scout Photographer Badge know your camera! welcome to the Single use cameras SLRs Digital cameras Single use cameras offer an inexpensive and ‘Single lens reflex’ cameras, often called SLRs, Digital cameras come in both compact and SLR exciting world of risk-free way to take great photos. They are built come in two main types - manual and auto-focus. formats. Rather than saving an image to film, complete with a film inside and once this is used SLRs give you greater artistic control as they can digital cameras save images onto memory cards. photography! up, the whole camera is sent for processing. They be combined with a vast range of interchangeable They have tiny sensors which convert an image are perfect for taking to places where you may lenses and accessories (such as lens filters). You electronically into ‘pixels’ (short for picture To successfully complete the Photographer Badge, be worried about losing or damaging expensive can also adjust almost every setting on the camera elements) which are put together to make up the you will need to learn the basic functions of a equipment (Scout camp for example) and you can yourself - aiding your photographic knowledge complete image. camera, how to use accessories, and how to care even get models suitable for underwater use - and the creative possibilities! for your equipment. You will also need to Capturing images this way means that as soon as perfect for taking to the beach! understand composition, exposure and depth of With manual SLRs, the photographer is in complete the picture is taken, you can view it on the LCD field, film types, how to produce prints and control - and responsible for deciding all the screen featured on most digital cameras. -

The Fascination of Flash Photography
The fascination of flash photography. 2016 / 2017 The fascination of flash photography “Paint” with the flash. Every subject has its own particular charm. The creative use of flash opens up numerous photographing possibilities, e.g. reducing the subject contrast, highlighting certain picture areas or getting rid of unwanted shadows. The first-class flash units from Metz offer natural colours and harmonious mood lighting – with every subject. Metz – always first class.. www.metz-mecatech.de picture: Geissler Dominik 2 — 3 Contents | A focus on quality 05 Tradition with a focus on the future 24 Every detail absolutely brilliant Contents 06 Your subject in the best 28 accessories possible light 32 Technical glossary 10 A lot of power for the best light 34 Technical specifications System flash units mecablitz mecablitz mecablitz 12 64 AF-1 digital 14 52 AF-1 digital 16 44 AF-2 digital mecablitz mecablitz mecablitz 18 M400 20 26 AF-2 22 36 AF-5 digital System flash units Specialist flash units mecablitz 18 M400 mecablitz mecablitz 23 24 AF-1 digital 26 15 MS-1 digital-Kit picture on title: Florian Gerlach Metz – always first class. www.metz-mecatech.de Metz mecatech – a focus on quality. The name Metz has been synonymous with professional flash units for decades. Our company’s exceptional reputation has been shaped by numerous technical innovations – such as the use of USB connections which allow flash units within the camera system to be updated for the latest camera model even after purchase. Today, we offer a diverse range of products from Whether light output, convenience of use or reliability, the convenient compact flashes right through to powerful handheld first-class quality of Metz flash units is unquestionable – as flash guns. -

[1C Darkroom Equipment Processing Equipment Ph-406
Ir TM 11-405 [1C DARKROOM EQUIPMENT PROCESSING EQUIPMENT PH-406 May 12, 1943 Generated on 2015-10-08 20:23 GMT / http://hdl.handle.net/2027/uc1.b3243829 Public Domain, Google-digitized / http://www.hathitrust.org/access_use#pd-google TM 11-405 Cl TECHNICAL MANUAL PHOTOGRAPHIC DARKROOM EQUIPMENT PROCESSING EQUIPMENT PH-406 CHANGES) (J^J> DEPARTMENT OF THE ARMY J No. 1 J WASHINGTON 25, D. C., 14 October 1948 TM 11-405, 12 May 1943, is changed as follows: 1. Purpose * * * Processing Equipment PH—406 from these negatives. Note (Added). Processing Equipment PH-406 procured on Order No. 11426- Phila-47-77 is identical to the equipment described in this manual, except as noted. 2. Components The components of Processing Equipment PH-406 are — * * * * * * * 1 Timer PH-126, automatic, electric; 1 Timer PH-426-A on Order No. 11426-Phila-47-77. *******2 11 14 3 Trays PH-161-A, stainless steel, by inches; Trays PH-161-A on Order No. 11426-PhiIa-47-77. 2 Trays PH-164-A, stainless steel, 14 by 17 inches; 1 Tray PH-164-A on Order No. 11426-Phila-47-77. *******12 Plates PH-152 or PH-152-A, ferrotype. *******1 1 Siphon PH-244; Siphon PH-244-A on Order No. 11426- Phila-47-77. * * * * v,T*fv" iT1-'5'*^ * . ^y.*! - v/^ 1 Accessory Group consisting of-— 2 Tongs PH-373-A, print, 8 inches; 2 tweezers, photo graphic, print, plastic, ?6 inches, on Order No. 11426- Phila-47-77. '--J \V/- ***** *f.-\ ,- * 3. Printer The projection printer * * * two enlarging lenses. -

Master Professional Portrait Lighting with These 20 Essential Studio Setups
LIGHTING GUIDE Master professional portrait lighting with these 20 essential studio setups REMBRANDT WITH A PORTALITE SOFTBOX REMBRANDT THROUGH AN UMBRELLA REMBRANDT WITH A HONEYCOMB GRID REMBRANDT WITH A SILVER UMBRELLA KIT: One D-lite RX4 head, one Clip-lock KIT: One D-lite RX4 head, KIT: One D-lite RX4 head, KIT: One D-lite RX4 head, Stand, one Portalite Softbox one Clip-lock Stand, one 16cm Reflector, one Clip-lock Stand, one 18cm Reflector one Clip-lock Stand, one 16cm Reflector, Position the light high and to the side to one Shoot-through Umbrella with Honeycomb one Silver Umbrella create a triangle on the model’s cheek. The Position the light high and to the side as with Position the light in the same manner as the Position the light in the same manner as the shadow of the nose should point towards the the ‘Rembrandt with a Portalite Softbox’ previous ‘Rembrandt’ techniques; the light previous ‘Rembrandt’ techniques. The light edge of the lips. The Portalite creates a soft setup. The light is slightly less contrasty, through the honeycomb grid is stronger and bouncing from the silver umbrella is more directional effect. because the light is less directional more dramatic. The grid makes it very easy direct and wraps around the features of the and there is always some reflection to direct the light on to the model and away face yet still creates the shadow from the from the studio surroundings. from the background, which becomes dark. nose towards the mouth. REMBRANDT SHORT REMBRANDT BROAD SPLIT SPLIT WITH FILL KIT: One D-lite RX4 head, one Clip-lock KIT: One D-lite RX4 head, one Clip-lock KIT: One D-lite RX4 head, one Clip-lock KIT: One D-lite RX4 head, one Clip-lock Stand, one Portalite Softbox Stand, one Portalite Softbox Stand, one Portalite Softbox Stand, one Portalite Softbox, one Use the principles of ‘Rembrandt’ lighting Use the principles of ‘Rembrandt’ lighting Position a light to one side of the model in small reflector to create the triangle of light on the face. -
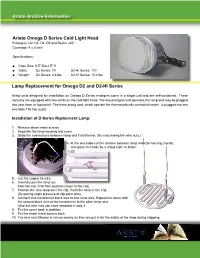
Lamp Replacement for Omega D2 and D2-HI Series Aristo Archive
Aristo Archive Information Aristo Omega D Series Cold Light Head Enlargers: D2, D3. D4, D5 and Besler 4x5* Coverage: 4 x 5 inch Specifications: ■ Case Size: 6.5” Dia x 5” H ■ Watts: D2 Series: 70 D2-HI Series: 110 ■ Weight: D2 Series: 4.8 lbs D2-HI Series: 15.4 lbs Lamp Replacement for Omega D2 and D2-HI Series Aristo units designed for installation on Omega D Series enlargers come in a single unit and are self-contained. These normally are equipped with two cords on the cold light head. The two pronged cord operates the lamp and may be plugged into your timer or footswitch. The three prong cord, which operate the thermostatically controlled heater, is plugged into any available 115 Vac outlet. Installation of D Series Replacment Lamp: 1. Remove sheet metal screws 2. Separate the lamp housing and cover 3. Undo the connections between lamp and Transformer. (By unscrewing the wire nuts.) 4. At the electrode-cut the silicone between lamp reflector housing (metal) and glass electrode by a sharp knife or blade. 5. Cut the copper tie wire. 6. Carefully pull the lamp out from the clip. (Pull from position closer to the clip) 7. Position the new lamp over the clip. Push the lamp in the Clip. (By putting slight pressure at clip point only) 8. Connect one transformer black wire to one lamp wire. Repeat the same with the second black wire of the transformer to the other lamp wire. (Use the wire nuts you have removed in step 3 8. Put the cover back in position. -

To Boldly Go: a Starters Guide to Hand Made and D-I-Y Films to Boldly Go: a Starters Guide to Hand Made and D-I-Y Films
To Boldly Go: a starters guide to hand made and d-i-y films To Boldly Go: a starters guide to hand made and d-i-y films What is most exciting about this type of filmmaking is not knowing exactly what will result. One then needs the lyrical, musical and cinematic taste to sculpt the result into a film which best demonstrates, exploits and celebrates the results of the experiments. If you stick with good ingredients you will in- evitably have happy results. Then again, not all experiments work but what you learn there can often be employed in a new direction or experiment to actually work. It’s also very important to have fun in and a love of the process. (Jeff Scher) It’s fun to handle film as a celluloid canvas rather than as a fragile This is a booklet for both those eager to begin in hand made film and carrier of images only to be handled by lab technicians. those who already started but are keen to know more. It signifies a hope You can experiment and create the most beautiful images ever. Helen Hill (1970 - 2007) and commitment to making sure these techniques, tricks and handy tips remain openly available to all who might need them. Let’s not keep any Recipes for Disaster: a handcrafted film cookbooklet secrets! These (chemical) receipts, printing processes, after dev. Effects, http://www.angoleiro.com/cine texts/recipes for disaster hill.pdf emulsion extras and celluloid experiments should be absolutely public. Let’s make hand-made d-i-y films! Let’s make a lot! Images and texts have been gathered, harvested, illegally used, replenished and inspired by a plethora of found sources including those of David Leis- ter, Greg Pope, Dirk De Bruyn, Maia Cybelle Carpenter, Frank Bruinsma, Steve Sanguedolce, Rebecca Moran, Jurgen Reble, Ben Russell, Jeff Scher and many many others. -

User Manual 2.3 MB
10M 25M 50M 75M 100M 10Y 25Y 50Y 75Y 100Y impact TM For EX-100A accessories and to see all of our lighting equipment, please visit our Web site. impactTM EX-100A Monolight www.impactstudiolighting.com INSTRUCTIONS Page 20 Page 1 (back cover) (front cover) 10M 25M 50M 75M 100M 10Y 25Y 50Y 75Y 100Y Thank you for your purchase of the Impact EX-100A Monolight. The One-Year Limited Warranty EX-100A Monolight is economical and lightweight, yet durable enough to give you many years of trouble-free service and enjoyment. Please read these operating instructions and safety precautions carefully before operating this equipment. Features • Three-stop range – full power to 1/8 power, steplessly • Built-in optical slave • Modeling lamp can be set to proportional or full power • Accepts Elinchrom-style reectors and head accessories (8-inch grid reector included) • Tactile, “grippy” feel that resists slipping, scratches, and shock damage • Commonly available 1/8˝ mini-plug sync input • Low 4.3V trigger voltage – safe for any camera’s circuitry Power Requirements This light comes in two models; one is designed for use with 110/120V AC power in the US and the other for 220V AC power in Europe. Neither model can be used outside of its native power region. Both are supplied with a three-prong, grounded plug. Do not attempt to defeat this safety feature. If necessary, use only grounded extension cords rated for six amps or greater. Warning There are no user-serviceable parts inside the unit. Only qualied service engineers should access the inside of the case (Danger – high-voltage parts inside). -

KINDERMANN .Ilm Developing System
KINDERMANN ilm Developing System E-1 Recognized worldwide as the finest quality film developing equipment made Made in Germany from Chrome-Nickel steel which is unbreakable, corrosion-proof, and easily brought to correct temperature. Complete system includes Reels, Tanks, and Reel Loaders. Designed to assure rapid, efficient, trouble-free film developing, with consistent, optimum results. Kindermann Stainless Steel Developing Tanks Kindermann Stainless Steel Developing Reels E ilm Developing System Heavy gauge chrome- Made of chrome-nickel steel: nickel steel is unbreakable unbreakable, chemical resistant, KINDERMANN and corrosion-proof. easy to clean. $ast, efficient, internal Only fractions of a millimeter of film edge flow-control saturates touches the reel, preventing damage. all the film instantly for Strong, rigid construction with center even development. core, keeps reel aligned and grips Leakproof, snug-fitting end of film for trouble-free loading. polyethylene tops for Can be used even when wet. immersion agitation. Master carton:10 Quick-pour top allows tanks to be rapidly filled Diameter Mfr# and emptied in daylight. EKM117 35mm Stainless Steel Reel 81.5mm 3117 Tanks come with cover and cap. Master carton:10 EKM119 120 Stainless Steel Reel 79mm 3119 Kindermann ReelInside ilm Loaders Capacity Dia. Hgt. Capacity Mfr# The quickest, safest method for loading EKM363 1-35mm 85mm 55mm 250ml 3363 film onto Kindermann reels. Prevents scratches, creases and EKM364 2-35mm or 1-120 85mm 88mm 450ml 3364 fingerprints. $ilm is guided evenly only at the outer edges of the perforation. EKM253 Replacement cover & cap for tanks 3253 Master carton:10 EKM875 Replacement tank cap only 9960 EKM326 35mm $ilm Loader 3326 EKM171 $ilter funnel to fit tank cover 3310 EKM329 120 $ilm Loader 3329 EKM3196 Replacement lift rod for tank EKM184 3196 EKM219 110 $ilm Loader 3219 PERSONAL SERVICE IS OUR #1 PRIORITY East: 111 Asia Place, Carlstadt, NJ 07072 (201) 939-7722 AX: (201) 939-7782 West: 4055 W. -

Backstage Lighting Terminology
Break-out: Adapter consisting of multiple receptacles (FM) wired to a single multipin (M) connector; may be a box or a cable assembly. Synonym: Break-out Box, Fan-out Burn Out: Failed lamp or color media that is burned through Channel: Specific control parameter encompassing single or multiple device attributes (lighting dimmers, audio signals, etc.) controlled as a unit Lighting and Electrics Terminology (A-Le) Channel Hookup: Paperwork designating the connection of Adapter: Electrical accessory that transitions between dimmer circuits to channels of control dissimilar connectors; may be a molded unit, box or cable assembly Circuit: Path for electricity to flow from the source, through a conductor, to a device(s) Amperes: Unit of measure for the quantity of electricity flowing in a conductor. Synonym: A, Amp, Current Circuit Breaker: Mechanical/Electrical device that is designed to automatically open (trip) if the current exceeds the rated Automated Luminaire: Lighting instrument with attributes level protecting the circuit; may be operated manually that are remotely controlled. Synonym: Automated Fixture, Synonym: Breaker, CB, OCPD, Overcurrent Protective Device Automated Light, Computerized Light, Intelligent Light, Motorized Light, Mover, Moving Light Color Extender: Top hat with color media holder. Synonym: Gel Extender Backlight: A lighting source that is behind the talent or subject from the viewers perspective. Synonym: Backs, Back Color Frame: Metal or heat resistant device that holds the Wash, Bx, Hair Light, Rim Light color media in front of a luminaire. Synonym: Gel Frame Balcony Rail: Lighting position mounted in front of or on the Color Media: Translucent material used to color light face of the balcony. -

I the Journal of \ Photographic Science
i- i The Journal of i \ Photographic Science SEP 15 1969 JULY / AUGUST 1969 • • ' !, ' • ,, , ': • I I VOLUME 17 NUMBER 4 • 0 • • • . ... CONTENTS PHOTOGRAPHY AND PHOTOGRAVURE: HISTORY OF PHOTOMECHANICAL REPRODUCTION E. OSTROFF THE RELATIONSHIP BETWEEN SPEED AND GRAIN SIZE G. C. FARNELL MICROIMAGE PHOTORESIST EVALUATION K. G. CLARK and R. G. TURNER HIGH SPEED PHOTOGRAPHIC STUDY OF LUBRICATED CONTACTS USING OPTICAL INTERFEROMETRY F. J. WESTLAKE and A. CAMERON FUTURE MEETINGS The Royal Photographic Society of Great Britain Ostroff: Photography and Photogravure 101 Photography and Photogravure: History of Photomechanical Reproduction EUGENE OSTROFF Curator of Photography, Smithsonian Institution, Washington, D.C. ABSTRACT. The first practicable photomechanical system-contact-screen photogravure-was invented in 1852 by W. H.F. Talbot of England. Many of the approaches introduced by Talbot are still used in current practice: contact cross-line "master" and "working" screens: metal plate etching through a bichromated gelatin emulsion: etching with ferric chloride solutions of different concentrations: and selective local etching for "retouching" purposes. To provide the tiny image ink-holding components in the printing plate, Talbot used fine gauze fabrics for the contact cross-line screens and fine resin particles (aquatint) applied as a powder or liquid. He also experimented with contact screens of ruled lines on paper: scored cartilage: waxed paper with scribed lines: aquatint pattern on paper and a blackened film with uniform grid of clear circular openings. INTRODUCTION By 1852, the year in which the first practicable photo- this coating was insufficient for camera exposures but ade- mechanical system was introduced, two different approaches quate for photogenic drawings (photograms) and contact had been devised-heliogravure (1826), and chemically etched printing.