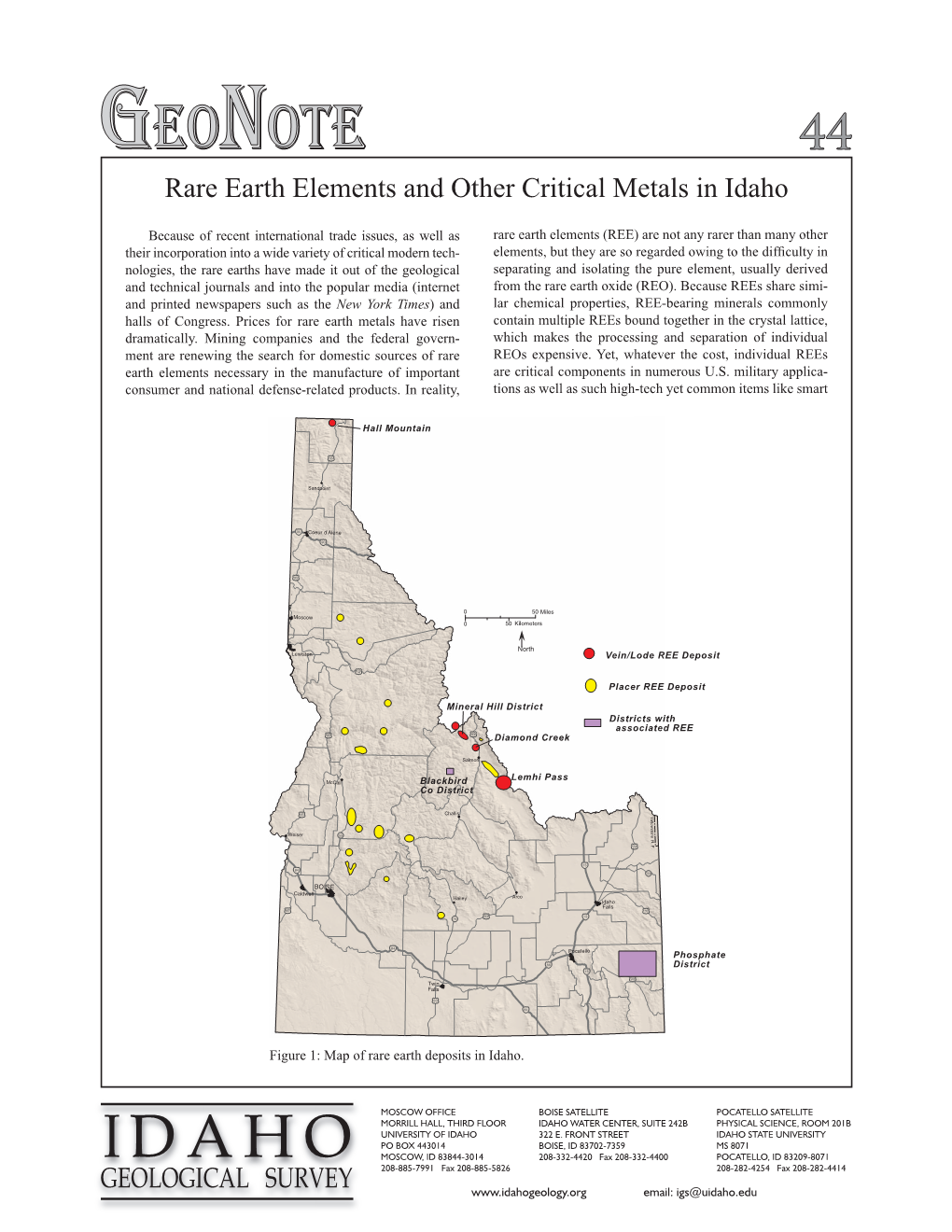
Rare Earth Elements and Other Critical Metals in Idaho
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crystal Chemistry of the Monazite and Xenotime Structures Yuxxnnc Nr
American Mineralogist, Volume 80, pages2I-26, 1995 Crystal chemistry of the monazite and xenotime structures YuxxnNc Nr, JonN M. Hucnns Department of Geology, Miami University, Oxford, Ohio 45056' U.S.A. ANrrrotvv N. M.q'nr,lNo 48 PageBrook Road, Carlisle, Massachusetts01741' U.S.A. Arsrnlcr Monazite and xenotime, the RE(PO,) dimorphs, are the most ubiquitous rare earth (RE) minerals, yet accuratestructure studiesof the natural phaseshave not been reported. Here we report the results of high-precision structure studies of both the natural phasesand the synthetic RE(PO4)phases for all individual stable rare earth elements. Monazite is monoclinic, P2r/n, and xenotime is isostructural with zircon (spacegroup 14r/amd)- Both atomic arrangementsare basedon [001] chains of intervening phosphate tetrahedra and RE polyhedra, with a REO, polyhedron in xenotime that accommodates the heavy lanthanides(Tb-Lu in the synthetic phases)and a REO, polyhedron in monazite that preferentially incorporatesthe larger light rare earth elements(Ia-Gd). As the struc- ture "transforms" from xenotime to monazite, the crystallographic properties are com- parable along the [001] chains, with structural adjustments to the different sizes of RE atoms occurring principally in (001). There are distinct similarities betweenthe structuresthat are evident when their atomic arrangementsare projected down [001]. In that projection, the chains exist i! (100) planes, with two planes per unit cell. In monazite the planes are offset by 2.2 A along [010], relative to those in xenotime, in order to accommodate the larger light RE atoms. The shift of the planes in monazite allows the RE atom in that phaseto bond to an additional 02' atom to complete the REO' polyhedron. -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

NEW MINERALS It Is Proposed Hereafter to Indicate In.A General Way the Classification of All New Minerals Recoided in This Department
JOURNAL MINERALOGICAL SOCIETY OF AMENICA 63 Dr. Kunz then spoke of the various city localities and the minerals found therein. He stated that the East Side, from 37 to 110 St., probably afforded the most specimens. The various tunnels and their minerals were spoken of. Capt. Miller called attention to the fine collection of Brooklyn Drift Minerals and Rocks in the collection of the Long Island Historical Society. Ife abo mentioned the occurrence of monazite and xenotime crystals, on the Speedway,Harlem River. Dr. Kunz emphasizedthe irnportance of complete records being kept of all finds. Tnou,q,s L Mrr,r,nn, SecretaryPro, Tem. NEW MINERALS It is proposed hereafter to indicate in.a general way the classification of all new minerals recoided in this department. Subdivision will be first into "families," of which nine may be recognized,as listed in the January number (Am. Min.6 (1), 12,1921). Eachfamilywillbe separatedinto "subfamilies " based on special features of composition. This arrangement is tentative and open to modification, and criticism of it will be welcome, [Eo.] FAMILY 2. SULFIDES, ETC. SosreMrr,v 3. Doust,u suLFrDEs oF METALSAND sEMr-METAr,s. I'LTRABASITE V. Rosrcxf and J. Srnnse-Btinu. Ultrabasit, ein neues Mineral aus Freiberg in Sachsen. (Ultrabasite, a new mineral from Freiberg, Saxony). Rozpr.Eeslcd Ako,il. Prag,25, No. 45, 1916;Z. Krgst. Min., 55,43H39, 1920, Neun: From its extremely basic chemical composition. Pnrsrcar, Pnopnnrrus Color black, somewhat grayish; luster metallic; streak black; cleavage none; fracture scaly, with somewhat greasy luster on the surface. H. : 5; sp. gr. -

Monazite, Rhabdophane, Xenotime & Churchite
Monazite, rhabdophane, xenotime & churchite: Vibrational spectroscopy of gadolinium phosphate polymorphs Nicolas Clavier, Adel Mesbah, Stephanie Szenknect, N. Dacheux To cite this version: Nicolas Clavier, Adel Mesbah, Stephanie Szenknect, N. Dacheux. Monazite, rhabdophane, xenotime & churchite: Vibrational spectroscopy of gadolinium phosphate polymorphs. Spec- trochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Elsevier, 2018, 205, pp.85-94. 10.1016/j.saa.2018.07.016. hal-02045615 HAL Id: hal-02045615 https://hal.archives-ouvertes.fr/hal-02045615 Submitted on 26 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Monazite, rhabdophane, xenotime & churchite : vibrational spectroscopy of gadolinium phosphate polymorphs N. Clavier 1,*, A. Mesbah 1, S. Szenknect 1, N. Dacheux 1 1 ICSM, CEA, CNRS, ENSCM, Univ Montpellier, Site de Marcoule, BP 17171, 30207 Bagnols/Cèze cedex, France * Corresponding author: Dr. Nicolas CLAVIER ICSM, CEA, CNRS, ENSCM, Univ Montpellier Site de Marcoule BP 17171 30207 Bagnols sur Cèze France Phone : + 33 4 66 33 92 08 Fax : + 33 4 66 79 76 11 [email protected] - 1 - Abstract : Rare-earth phosphates with the general formula REEPO4·nH2O belong to four distinct structural types: monazite, rhabdophane, churchite, and xenotime. -

Mineral Collecting Sites in North Carolina by W
.'.' .., Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie RUTILE GUMMITE IN GARNET RUBY CORUNDUM GOLD TORBERNITE GARNET IN MICA ANATASE RUTILE AJTUNITE AND TORBERNITE THULITE AND PYRITE MONAZITE EMERALD CUPRITE SMOKY QUARTZ ZIRCON TORBERNITE ~/ UBRAR'l USE ONLV ,~O NOT REMOVE. fROM LIBRARY N. C. GEOLOGICAL SUHVEY Information Circular 24 Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie Raleigh 1978 Second Printing 1980. Additional copies of this publication may be obtained from: North CarOlina Department of Natural Resources and Community Development Geological Survey Section P. O. Box 27687 ~ Raleigh. N. C. 27611 1823 --~- GEOLOGICAL SURVEY SECTION The Geological Survey Section shall, by law"...make such exami nation, survey, and mapping of the geology, mineralogy, and topo graphy of the state, including their industrial and economic utilization as it may consider necessary." In carrying out its duties under this law, the section promotes the wise conservation and use of mineral resources by industry, commerce, agriculture, and other governmental agencies for the general welfare of the citizens of North Carolina. The Section conducts a number of basic and applied research projects in environmental resource planning, mineral resource explora tion, mineral statistics, and systematic geologic mapping. Services constitute a major portion ofthe Sections's activities and include identi fying rock and mineral samples submitted by the citizens of the state and providing consulting services and specially prepared reports to other agencies that require geological information. The Geological Survey Section publishes results of research in a series of Bulletins, Economic Papers, Information Circulars, Educa tional Series, Geologic Maps, and Special Publications. -

Preliminary Estimates of the Quantities of Rare-Earth Elements Contained in Selected Products and in Imports of Semimanufactured Products to the United States, 2010
Preliminary Estimates of the Quantities of Rare-Earth Elements Contained in Selected Products and in Imports of Semimanufactured Products to the United States, 2010 By Donald I. Bleiwas and Joseph Gambogi Open-File Report 2013–1072 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2013 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order other USGS information products, visit http://store.usgs.gov Suggested citation: Bleiwas, D.I., and Gambogi, Joseph, 2013, Preliminary estimates of the quantities of rare-earth elements contained in selected products and in imports of semimanufactured products to the United States, 2010: U.S. Geological Survey Open–File Report 2013–1072, 14 p., http://pubs.usgs.gov/of/2013/1072/. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner. Cover. Left: Aerial photograph of Molycorp, Inc.’s Mountain Pass rare-earth oxide mining and processing facilities in Mountain Pass, California. -

Transmittal of Draft EIR for Molycorp Mountain Pass Mine Expansion, for Review and Comment
COUNTY OF SAN BERNARDINO PLANNING DEPARTMENT I,regular-! I PUBLIC WORKS GROUP -- W&- Sw1. NENNO lorth Arrowhead Avenue * San Bernardino, CA 924154182 * (909) 3874131 VALERY PILMER Director of Planning December 9, 1996No. 909) 3873223 IY RESPONSIBLE AND TRUSTEE AGENCIES INTERESTED ORGANIZATIONS AND INDIVIDUALS RE: NOTICE OF AVAILABILITY FOR THE DRAFT EIR ON THE MOLYCORP MOUNTAIN PASS MINE EXPANSION Dear Reader/Reviewer: Enclosed for your review and comment is the Draft EIR for the Molycorp Mountain Pass Mine Expansion. The purpose of the document is to identify and describe the probable environmental impacts that would result from the proposed expansion of Molycorp's existing mine and processing plant complex located at Mountain Pass, California. Mountain Pass is within the unincorporated portion San Bernardino County along Interstate 15 approximately 30 miles northeast of Baker and approximately 14 miles southwest of the Nevada stateline. The proposed quarry and waste rock areas would add 696 acres of disturbance to the existing mine site, resulting in a total disturbed area of approximately 1,044 acres. sag_> This document has been prepared to meet the State requirements of the California Environmental Quality Act. The Draft EIR has been prepared under the supervision of the County of San Bernardino Planning Department. The public comment period will end on January 27, 1997. Written comments should be addressed to: County of San Bemnardino- Planning Dgparnn 385 N. Arrowhead Avenue, Third Floor San Bernardino, CA 92415-0182 Attn: Randy Scott Sincerely, Randy Scottjlanning Manager, San Berardo County Planning Department ~-! - nLA ,;;K Scr.'Eperviscs .,* 1:,. 3 - -, I- . 1 12 4~ ..!A','V34-' TUrCCI Vi~i D;s~ri:n, BARBA~RA CPANM FURtOPAN . -

Producers Case Study the Changing
Producers Case Study The Changing Geography of Rare Earth Element Production Introduction The locations where rare earth elements are produced changed repeatedly throughout the 1900s and early 2000s. This variation suggests that production is not determined primarily by the geographic location of rare earth ores. Instead, the location of mines and separation plants is driven by a combination of market prices, government policies, and the actions of producers and manufacturers. As a result where and how rare earth elements are produced could change in the future. Initial Rare Earth Metal Production Although a mineral containing rare earth elements was identified in Sweden in 1788, it took more than 100 years for the first significant industrial product to be made using the rare earths. In the 1890s chemist Carl Auer von Welsbach developed a mantle made of a mixture of 99% thorium and 1% cerium for use with gas streetlights. More than five billion mantles were sold worldwide through the 1930s. Welsbach also developed mischmetal, a mixture of rare earth elements cerium, lanthanum, neodymium, and praseodymium. When alloyed with iron, mischmetal produced a metal that sparked when struck. It was widely used in pocket cigarette lighters as well as automobile ignition switches. The Welsbach Company mined rare earth deposits located in coastal sands in Brazil, India, and North Carolina. India’s coastal sands are also rich in the radioactive element thorium, which is not a rare earth element. India banned the export of these sands in 1948 to preserve its thorium supply for a potential nuclear energy program. At that time the price of rare earth metals spiked until newly established mining locations briefly made South Africa the world’s leading exporter of rare earth ores in the 1950s. -

4-1 Section 4. Transportation of Radioactive Materials
SECTION 4. TRANSPORTATION OF RADIOACTIVE MATERIALS PART A. GENERAL RH-3000. Authority. Act 8 of Second Extraordinary Session of 1961, as amended. RH-3001. Effective Date. The provisions of these Regulations shall become operative on the effective date of an agreement executed by the State of Arkansas and the Federal Government under the provisions of Section 274 of the Atomic Energy Act of 1954 as amended (73 STAT. 689). RH-3002. Purpose and Scope. a. This Section establishes requirements for packaging, preparation for shipment, and transportation of licensed material. b. The packaging and transport of licensed material are also subject to the regulations of other agencies (e.g., the U.S. Department of Transportation, the U.S. Nuclear Regulatory Commission, and the U.S. Postal Service) having jurisdiction over means of transport. The requirements of this Section are in addition to, and not in substitution for, other requirements. c. The regulations in this Section apply to any licensee authorized by specific or general license issued by the Department to receive, possess, use, or transfer licensed material, if the licensee delivers that material to a carrier for transport, transports the material outside the site of usage as specified in the Department license, or transports that material on public highways. No provision of this Section authorizes the possession of licensed material. d. 1. Exemptions from Section 4 requirements are specified in Part C of this Section. General licenses for which no NRC package approval is required are issued in RH-3304. through RH-3306. The general license in RH-3301. requires that an NRC Certificate of Compliance or other package approval be issued for the package to be used under this general license. -

Critical Materials and US Import Reliance
Testimony Critical Materials and U.S. Import Reliance Recent Developments and Recommended Actions Richard Silberglitt CT-485 Testimony presented before House Natural Resources Committee, Subcommittee on Energy and Mineral Resources on December 12, 2017. For more information on this publication, visit www.rand.org/pubs/testimonies/CT485.html Testimonies RAND testimonies record testimony presented or submitted by RAND associates to federal, state, or local legislative committees; government-appointed commissions and panels; and private review and oversight bodies. Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2017 RAND Corporation is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions.html. www.rand.org Critical Materials and U.S. Import Reliance: Recent Developments and Recommended Actions Testimony of Richard Silberglitt1 The RAND Corporation2 Before the Committee on Natural Resources Subcommittee on Energy and Mineral Resources United States House of Representatives December 12, 2017 hank you Chairman Gosar, Ranking Member Lowenthal, and distinguished members of the Subcommittee for inviting me to testify today. My testimony is based on the results of a 2013 study conducted by the RAND Corporation at the request of the National T 3 Intelligence Council, taking into account relevant developments and data since the publication of that report. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table.