Thioredoxin Targets Fundamental Processes in a Methane-Producing Archaeon, Methanocaldococcus Jannaschii
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

Table S4. List of Enzymes Directly Involved in the Anti-Oxidant Defense Response
Table S4. List of Enzymes directly involved in the anti-oxidant defense response. Gene Name Gene Symbol Classification/Pathway 6-phosphogluconate dehydrogenase 6PGD NADPH regeneration/Pentose Phosphate Glucose-6-phosphate dehydrogenase G6PD NADPH regeneration/Pentose Phosphate Isocitrate Dehydrogenase 1 IDH1 NADPH regeneration/Krebs Isocitrate Dehydrogenase 2 IDH2 NADPH regeneration/Krebs Malic Enzyme 1 ME1 NADPH regeneration/Krebs Methylenetetrahydrofolate dehydrogenase 1 MTHFD1 NADPH regeneration/Folate Methylenetetrahydrofolate dehydrogenase 2 MTHFD2 NADPH regeneration/Folate Nicotinamide Nucleotide Transhydrogenase NNT NADPH regeneration/NAD Catalase CAT Antioxidants/Catalses/free radical detoxification Glutamate-cysteine ligase catalytic subunit GCLC Antioxidants/Glutathione synthesis Glutamate-cysteine ligase modifier subunit GCLM Antioxidants/Glutathione synthesis Glutathione peroxidase1 GPx1 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase2 GPx2 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase3 GPx3 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase4 GPx4 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase5 GPx5 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase6 GPx6 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase7 GPx7 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione S-transferase -
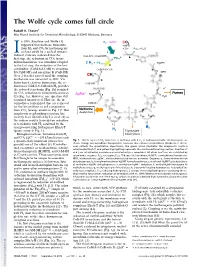
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

Inhibitory Effects of Some Flavonoids on Thioredoxin Reductase Purified from Chicken Liver ABSTRACT INTRODUCTION
Brazilian Journal of Poultry Science Revista Brasileira de Ciência Avícola Inhibitory Effects of Some Flavonoids on ISSN 1516-635X 2019 / v.21 / n.2 / 001-008 Thioredoxin Reductase Purified from Chicken http://dx.doi.org/10.1590/1806-9061-2018-0982 Liver Original Article Author(s) ABSTRACT Türkoğlu E.AI https://orcid.org/0000-0001-7850-6456 Thioredoxin reductases (TrxRs) are selenocysteine-containing Kuzu MII https://orcid.org/0000-0002-1375-7673 flavoenzymes that reduce Trxin NADPH-dependent manner. In the Ayasan TIII https://orcid.org/0000-0001-7397-6483 view of the direct vital role of TrxR in a wide range of biochemical and IV Inci H https://orcid.org/0000-0002-9791-0435 physiological processes, methods to inhibit this enzyme are clinically Eratak SVV https://orcid.org/0000-0003-3788-8704 important. TrxR has recently emerged as a new candidate in anticancer I Department of Pharmaceutical Biotechnology, drug investigations because of overexpression in tumorous cells. In this Faculty of Pharmacy, University of Health Sciences, Istanbul 34668, Turkey. study, TrxR from chick liver was purified 94.6-fold with a yield of 4.86% II Deparment of Chemistry, Faculty of Science and a specific activity of 0.19 EU/mg. K and V values of TrxR for and Letters, Ağrı İbrahim Çeçen University, Ağrı M max 04100, Turkey. DTNB were calculated as 0.9 mM and 0,03 EU/mL, respectively. Then, III East Mediterranean Agricultural Research Institute, Karatas Road, Adana 01321, Turkey. the effects of the flavonoids hesperidin, naringenin, chlorogenic acid, IV Department of Animal Science, Faculty of ferulic acid, naringin, 3,4-dihydoxybenzoic acid, and ellagic acid on the Agriculture, Bingöl University, Bingöl 12000, Turkey. -

Genomic Insights Into the Uncultured Genus &Lsquo
The ISME Journal (2014) 8, 2463–2477 & 2014 International Society for Microbial Ecology All rights reserved 1751-7362/14 www.nature.com/ismej ORIGINAL ARTICLE Genomic insights into the uncultured genus ‘Candidatus Magnetobacterium’ in the phylum Nitrospirae Wei Lin1,2,7, Aihua Deng3,7, Zhang Wang4, Ying Li2,5, Tingyi Wen3, Long-Fei Wu2,6, Martin Wu4 and Yongxin Pan1,2 1Biogeomagnetism Group, Paleomagnetism and Geochronology Laboratory, Key Laboratory of the Earth’s Deep Interior, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China; 2France-China Bio-Mineralization and Nano-Structures Laboratory, Chinese Academy of Sciences, Beijing, China; 3CAS Key Laboratory of Microbial Physiological and Metabolic Engineering, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; 4Department of Biology, University of Virginia, Charlottesville, VA, USA; 5State Key Laboratory of Agro-Biotechnology and Laboratoire International Associe Franco-Chinois de Bio-Mineralisation et Nano-Structures, College of Biological Sciences, China Agricultural University, Beijing, China and 6Laboratoire de Chimie Bacte´rienne, Aix-Marseille Universite´, CNRS, Marseille Cedex 20, France Magnetotactic bacteria (MTB) of the genus ‘Candidatus Magnetobacterium’ in phylum Nitrospirae are of great interest because of the formation of hundreds of bullet-shaped magnetite magneto- somes in multiple bundles of chains per cell. These bacteria are worldwide distributed in aquatic environments and have important roles in the biogeochemical cycles of iron and sulfur. However, except for a few short genomic fragments, no genome data are available for this ecologically important genus, and little is known about their metabolic capacity owing to the lack of pure cultures. Here we report the first draft genome sequence of 3.42 Mb from an uncultivated strain tentatively named ‘Ca. -

Supplemental Methods
Supplemental Methods: Sample Collection Duplicate surface samples were collected from the Amazon River plume aboard the R/V Knorr in June 2010 (4 52.71’N, 51 21.59’W) during a period of high river discharge. The collection site (Station 10, 4° 52.71’N, 51° 21.59’W; S = 21.0; T = 29.6°C), located ~ 500 Km to the north of the Amazon River mouth, was characterized by the presence of coastal diatoms in the top 8 m of the water column. Sampling was conducted between 0700 and 0900 local time by gently impeller pumping (modified Rule 1800 submersible sump pump) surface water through 10 m of tygon tubing (3 cm) to the ship's deck where it then flowed through a 156 µm mesh into 20 L carboys. In the lab, cells were partitioned into two size fractions by sequential filtration (using a Masterflex peristaltic pump) of the pre-filtered seawater through a 2.0 µm pore-size, 142 mm diameter polycarbonate (PCTE) membrane filter (Sterlitech Corporation, Kent, CWA) and a 0.22 µm pore-size, 142 mm diameter Supor membrane filter (Pall, Port Washington, NY). Metagenomic and non-selective metatranscriptomic analyses were conducted on both pore-size filters; poly(A)-selected (eukaryote-dominated) metatranscriptomic analyses were conducted only on the larger pore-size filter (2.0 µm pore-size). All filters were immediately submerged in RNAlater (Applied Biosystems, Austin, TX) in sterile 50 mL conical tubes, incubated at room temperature overnight and then stored at -80oC until extraction. Filtration and stabilization of each sample was completed within 30 min of water collection. -

Hydrogenases of Methanogens
ANRV413-BI79-18 ARI 27 April 2010 21:0 Hydrogenases from Methanogenic Archaea, Nickel, a Novel Cofactor, and H2 Storage Rudolf K. Thauer, Anne-Kristin Kaster, Meike Goenrich, Michael Schick, Takeshi Hiromoto, and Seigo Shima Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany; email: [email protected] Annu. Rev. Biochem. 2010. 79:507–36 Key Words First published online as a Review in Advance on H2 activation, energy-converting hydrogenase, complex I of the March 17, 2010 respiratory chain, chemiosmotic coupling, electron bifurcation, The Annual Review of Biochemistry is online at reversed electron transfer biochem.annualreviews.org This article’s doi: Abstract 10.1146/annurev.biochem.030508.152103 Most methanogenic archaea reduce CO2 with H2 to CH4. For the Copyright c 2010 by Annual Reviews. activation of H2, they use different [NiFe]-hydrogenases, namely All rights reserved energy-converting [NiFe]-hydrogenases, heterodisulfide reductase- 0066-4154/10/0707-0507$20.00 associated [NiFe]-hydrogenase or methanophenazine-reducing by University of Texas - Austin on 06/10/13. For personal use only. [NiFe]-hydrogenase, and F420-reducing [NiFe]-hydrogenase. The energy-converting [NiFe]-hydrogenases are phylogenetically related Annu. Rev. Biochem. 2010.79:507-536. Downloaded from www.annualreviews.org to complex I of the respiratory chain. Under conditions of nickel limitation, some methanogens synthesize a nickel-independent [Fe]- hydrogenase (instead of F420-reducing [NiFe]-hydrogenase) and by that reduce their nickel requirement. The [Fe]-hydrogenase harbors a unique iron-guanylylpyridinol cofactor (FeGP cofactor), in which a low-spin iron is ligated by two CO, one C(O)CH2-, one S-CH2-, and a sp2-hybridized pyridinol nitrogen. -

Sequencing, Assembly, and Annotation of the Kaistella Koreensis Genome and Comparison to Closely Related Organisms
Sequencing, Assembly, and Annotation of the Kaistella koreensis Genome and Comparison to Closely Related Organisms Presented to the faculty of Lycoming College in partial fulfillment of the requirements for Departmental Honors in Biology by Timothy Hostelley Lycoming College April 22, 2013 Approved by: (Signature) (Signature) (Signature) (Signature) Abstract Advances in DNA sequencing technology have made DNA sequencing cheaper and more efficient. As a result, there has been an enormous increase in the number of genomes being sequenced. The sequence data can be assembled into complete genomes and annotated in order to reveal information about the organism’s physiology. In this study the DNA of the bacterium Kaistella koreensis was sequenced and assembled into 578 contigs, these contigs were then uploaded to Rapid Annotation Using Subsystems Technology for annotation in order to compute the Average Nucleotide Identity between K. koreensis and closely related organisms in order to dispute the reclassification of K. koreensis as Chryseobacterium koreense. Phenotypic tests including Biolog GenII, API ZYM, and Fatty Acid Methyl Ester analysis were also done in order to supplement the ambiguous results of the ANI. The results of these tests reveal a number of significant differences between K. koreensis and its closest related neighbors that suggests that K. koreensis does not belong in the Chryseobacterium genus or the closely related Lycomia genus. Instead, K. koreensis should be reclassified back to its original classification in the Kaistella genus. This would dispute the proposal made by Kämpfer et al. to reclassify Kaistella koreensis into the Chryseobacterium genus. 1 Introduction DNA sequencing has rapidly evolved from the earliest sequencing efforts using only whole genome shotgun-cloning based sequencing of the 1990’s, to the further advances in Sanger sequencing in the early 2000’s. -

Emerging Players in the Regulation of Protein S-Nitrosation in Plants
plants Review Thioredoxins: Emerging Players in the Regulation of Protein S-Nitrosation in Plants Tereza Jedelská , Lenka Luhová and Marek Petˇrivalský * Department of Biochemistry, Faculty of Science, Palacký University, Šlechtitel ˚u27, 78371 Olomouc, Czech Republic; [email protected] (T.J.); [email protected] (L.L.) * Correspondence: [email protected] Received: 17 August 2020; Accepted: 22 October 2020; Published: 24 October 2020 Abstract: S-nitrosation has been recognized as an important mechanism of ubiquitous posttranslational modification of proteins on the basis of the attachment of the nitroso group to cysteine thiols. Reversible S-nitrosation, similarly to other redox-based modifications of protein thiols, has a profound effect on protein structure and activity and is considered as a convergence of signaling pathways of reactive nitrogen and oxygen species. This review summarizes the current knowledge on the emerging role of the thioredoxin-thioredoxin reductase (TRXR-TRX) system in protein denitrosation. Important advances have been recently achieved on plant thioredoxins (TRXs) and their properties, regulation, and functions in the control of protein S-nitrosation in plant root development, translation of photosynthetic light harvesting proteins, and immune responses. Future studies of plants with down- and upregulated TRXs together with the application of genomics and proteomics approaches will contribute to obtain new insights into plant S-nitrosothiol metabolism and its regulation. Keywords: denitrosation; -

Xuejun Yu Dissertation Final
UNIVERSITY OF CALIFORNIA RIVERSIDE Conversion of Carbon Dioxide to Formate by a Formate Dehydrogenase from Cupriavidus necator A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Bioengineering by Xuejun Yu September 2018 Dissertation Committee: Dr. Ashok Mulchandani, Co-Chairperson Dr. Xin Ge, Co-Chairperson Dr. Russ Hille Copyright by Xuejun Yu 2018 The Dissertation of Xuejun Yu is approved: Committee Co-Chairperson Committee Co-Chairperson University of California, Riverside ACKNOWLEDGEMENTS I would like to express sincere appreciation to my advisor, Professor Ashok Mulchandani, for accepting me to be his student when I was suffering. Thank you very much for your delicate guidance, support and encouragement during the past years. You not only guide me on my PhD study, but also help me to be mature on my personality. From bottom of my heart, I feel very lucky to have you as professor. Also, many thanks go to my other committee members. Professor Xin Ge acted as my co-advisor and provided many valuable comments on my molecular cloning work. Professor Russ Hille has also provided insights, encouragements and advices as a member of my committee members. I have learned so many important insights from our meetings and discussions and thank you for bringing me into the molybdenum/tungsten enzyme conference. I am also thankful to the past and current group members, colleagues and friends - Dimitri Niks, Pankaj Ramnani, Feng Tan, Rabeay Hassan, Trupti Terse, Thien-Toan Tran, Claudia Chaves, Jia-wei Tay, Hui Wang, Pham Tung, Hilda Chan, Yingning Gao and Tynan Young. -

DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents
molecules Review DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents Maria Valeria Raimondi 1,*,† , Ornella Randazzo 1,†, Mery La Franca 1 , Giampaolo Barone 1 , Elisa Vignoni 2, Daniela Rossi 2 and Simona Collina 2,* 1 Department of Biological, Chemical and Pharmaceutical Sciences and Technologies (STEBICEF), University of Palermo, via Archirafi 32, 90123 Palermo, Italy; [email protected] (O.R.); [email protected] (M.L.F.); [email protected] (G.B.) 2 Drug Sciences Department, Medicinal Chemistry and Pharmaceutical Technology Section, University of Pavia, via Taramelli 12, 27100 Pavia, Italy; [email protected] (E.V.); [email protected] (D.R.) * Correspondence: [email protected] (M.V.R.); [email protected] (S.C.); Tel.: +390-912-389-1915 (M.V.R.); +390-382-987-379 (S.C.) † These Authors contributed equally to this work. Academic Editors: Simona Collina and Mariarosaria Miloso Received: 25 February 2019; Accepted: 20 March 2019; Published: 22 March 2019 Abstract: Dihydrofolate reductase inhibitors are an important class of drugs, as evidenced by their use as antibacterial, antimalarial, antifungal, and anticancer agents. Progress in understanding the biochemical basis of mechanisms responsible for enzyme selectivity and antiproliferative effects has renewed the interest in antifolates for cancer chemotherapy and prompted the medicinal chemistry community to develop novel and selective human DHFR inhibitors, thus leading to a new generation of DHFR inhibitors. This work summarizes the mechanism of action, chemical, and anticancer profile of the DHFR inhibitors discovered in the last six years. New strategies in DHFR drug discovery are also provided, in order to thoroughly delineate the current landscape for medicinal chemists interested in furthering this study in the anticancer field.