Gelgreen® Product Information Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gelred® and Gelgreen® Safety Report
Safety Report for GelRed® and GelGreen® A summary of mutagenicity and environmental safety test results from three independent laboratories for the nucleic acid gel stains GelRed® and GelGreen® www.biotium.com General Inquiries: [email protected] Technical Support: [email protected] Phone: 800-304-5357 Conclusion Overview GelRed® and GelGreen® are a new generation of nucleic acid gel stains. Ethidium bromide (EB) has been the stain of choice for nucleic acid gel They possess novel chemical features designed to minimize the chance for staining for decades. The dye is inexpensive, sufficiently sensitive and very the dyes to interact with nucleic acids in living cells. Test results confirm that stable. However, EB is also a known powerful mutagen. It poses a major the dyes do not penetrate latex gloves or cell membranes. health hazard to the user, and efforts in decontamination and waste disposal ultimately make the dye expensive to use. To overcome the toxicity problem In the AMES test, GelRed® and GelGreen® are noncytotoxic and of EB, scientists at Biotium developed GelRed® and GelGreen® nucleic acid nonmutagenic at concentrations well above the working concentrations gel stains as superior alternatives. Extensive tests demonstrate that both used in gel staining. The highest dye concentrations shown to be non-toxic dyes have significantly improved safety profiles over EB. and non-mutagenic in the Ames test for GelRed® and GelGreen® dyes are 18.5-times higher than the 1X working concentration used for gel casting, and 6-times higher than the 3X working concentration used for gel staining. This Dye Design Principle is in contrast to SYBR® Safe, which has been reported to show mutagenicity At the very beginning of GelRed® and GelGreen® development, we made a in several strains in the presence of S9 mix (1). -

Gelred™& Gelgreen™
Glowing Products for ScienceTM GelRed™& GelGreen™ www.biotium.com Safe and sensitive nucleic acid gel stains designed to replace the highly toxic ethidium bromide (EtBr). Developed by G scientists at Biotium, GelRed™ and GelGreen™ are superior to EtBr and other SYBR® Safe GelRed™ GelGreen™ EtBr alternatives by having a combination of low toxicity, high sensitivity and exceptional stability. EtBr has been the predominant dye used for nucleic acid gel staining for decades mutagenic chemical. The safety hazard and costs associated with decontamination and waste disposal can ultimately make the dye expensive and inconvenient to use. For this reason, alternative gel stains, such as SYBR® dyes, have become commercially available Figure 2. GelRed™ and GelGreen™ gel stains are safer because they cannot penetrate cell in recent years. While these alternative dyes have reduced mutagenicity membranes to bind DNA in living cells. HeLa cells were incubated at 37oC with 1X SYBR® Safe, sensitivity and stability. For example, SYBR® Safe has very limited sensitivity while GelGreen™ or GelRed™, respectively. Images were taken following incubation with dye for 30 SYBR® Green and SYBR® Gold are much less stable than EtBr. SYBR® dyes also enter SYBR® Safe rapidly entered cells and stained nuclei. GelRed™ and GelGreen™ were unable cells rapidly to stain mitochondria and nuclear DNA, making it more likely for the dyes to be harmful to cells. Indeed, SYBR® Green I has been shown to strongly potentiate DNA was observed in dead cells present sporadically in the cultures, as is observed with other non- mutation caused by UV light and other mutagens (Ohta, et al. -

UBC's Safer Alternatives to Ethidium Bromide
Risk Management Services Environmental Services www.riskmanagement.ubc.ca/environment Safer Alternatives to Ethidium Bromide Different types of dyes are used to stain nucleic acids in the preparation and use of electrophoresis gels. The hazard properties of various products, and hence the disposal requirements, are very different. While some products are completely safe or less toxic, others are mutagenic and require special handling and disposal procedures. As new products become available it is important to clarify the hazard properties and disposal requirements of these dyes. [Photo from: http://en.wikipedia.org/wiki/Ethidium_bromide] Non-Mutagenic Dyes SYBR®Safe, GelRed™, GelGreen™, and EvaGreen®. Independent licensed testing laboratories have determined in Ames tests that these dyes are non-mutagenic. Mutagenic Dyes The following dyes have been determined to have mutagenic and/or toxic properties: Ethidium Bromide, Methylene Blue, Crystal Violet, Propidium Iodide, Acridine Orange, SYBR®Green I, SYBR®Green II, SYBR®Gold and GelStar™. All gels containing these dyes, unwanted dye stock solutions, and all contaminated debris must be handled and disposed as hazardous waste. For details refer to the Laboratory Pollution Prevention and Hazardous Waste Management Manual. Learn More (below is a comparison of some commonly used DNA stains): Ethidium Bromide Ethidium bromide (3,8-Diamino-5-ethyl-6- phenylphenanthridinium bromide) is the classic DNA stain. Ethidium bromide (EtBr) is a flat molecule that fits between adjacent base pairs (intercalates) in the DNA double helix. It has UV absorbance maxima at 300 and 360nm, and can also absorb energy from nucleotides excited at 260nm. The absorbed energy is emitted as orange/yellow light at 590nm. -

Gelred Flyer
Glowing Products for ScienceTM GelRedTM & GelGreenTM Environmentally safe and ultra-sensitive nucleic acid gel stains for replacing EtBr April 6, 2009 GelRed Is Superior to EtBr GelRed EtBr FEATURES Safer than EtBr Shown by Ames test and other tests to be nonmutagenic and noncytotoxic. Easy disposal Passed environmental safety tests for direct disposal down the drain or in regular trash. Ultra-sensitive Much more sensitive than EtBr and SYBR Safe. Extremely stable Available in water, stable at room temperature for long-term storage and microwavable. Figure 1. Comparison of GelRed and ethidium bromide (EtBr) in precast gel staining using 1% agaose gel in TBE buffer. Two-fold serial dilutions of 1 kb Plus DNA Ladder from Invitrogen were loaded onto each gel in 4 lanes in the amounts of 200 ng, 100 ng, 50 ng and 25 ng, respectively, from left to right. Flexible for different procedures Gels were imaged using a 300-nm transilluminator and photographed with an EtBr filter and Polaroid Can be used for either precast or post gel staining 667 black-and-white print films. Simple to use Very simple procedures for precast and post gel stainings. Perfect Compatibility with a Standard UV Transilluminator or a Gel Reader with GelGreen Is Simply Unmatched Visible Light Excitation by SYBR Safe GelRed replaces EtBr with no optical setting change; GelGreen replaces SYBR or GelStar with GelGreen SYBR Safe no optical setting change(See Figure 3 for spectra). GelGreen Excitation Gel Green Emission GelRed Emission GelRed Excitation 200 300 400 500 600 700 Wavelength (nm) Figure 2. Comparison of GelGreen and SYBR Safe in post gel staining using 1% aga- rose gel in TBE buffer. -

Comparison of Nucleic Acid Gel Stains Cell Permeability, Safety, And
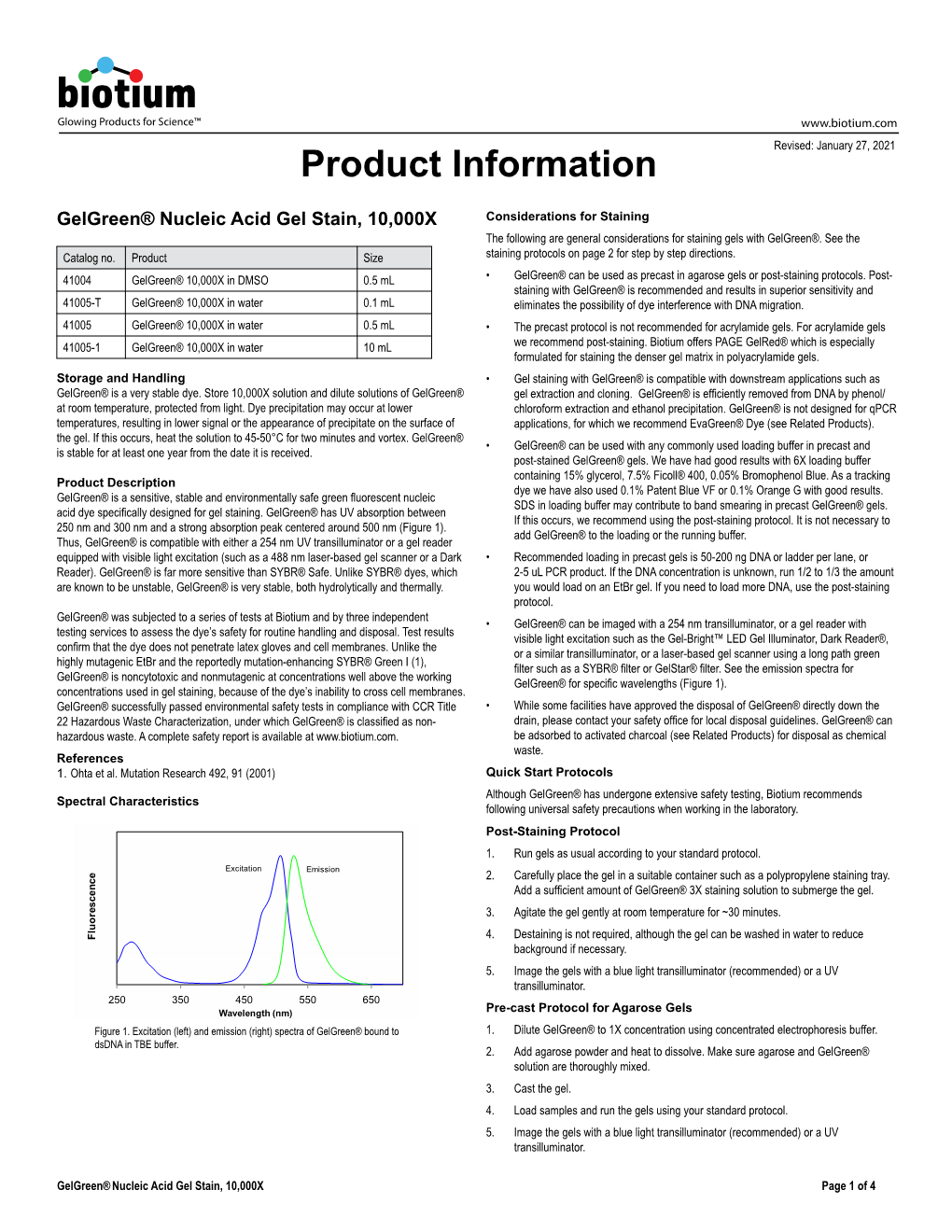
Glowing Products for Science™ www.biotium.com February 1, 2017 Comparison of Nucleic Acid Gel Stains Cell permeability, safety, and sensitivity of ethidium bromide alternatives Mikhail Guzaev, Xue Li, Candice Park, Wai-Yee Leung, and Lori Roberts Biotium, Inc., Fremont, CA Introduction Chemical analysis Nucleic acid gel stains were analyzed by thin layer chromatography (TLC). Dye Ethidium bromide has been used for decades to detect nucleic acids in agarose gels absorbance spectra were measured using a Beckman Coulter DU-800 UV-visible because it is inexpensive, sensitive enough for routine applications, and simple to spectrophotometer. Dye fluorescence emission spectra were measured in the presence use. However, because ethidium bromide has been shown to be mutagenic in the of double stranded DNA using a Hitachi F-4500 fluorescence spectrophotometer. Mass Ames test, it is potentially hazardous to laboratory workers and the environment, and spectrometry (LC-MS) was performed on an Agilent 6130 Quadrupole System. C18 institutions commonly require it to be disposed of as hazardous waste. Because of this, reverse phase HPLC was performed on an Agilent 1100 Series System. DAPI (catalog many users seek to find safer alternatives to ethidium bromide for gel electrophoresis. no. 40011), propidium iodide (catalog no. 40016), and acridine orange (catalog no. Biotium developed red fluorescent GelRed™ and green fluorescent GelGreen™ as 40039) from Biotium were used as reference dyes. Acridine orange from Sigma-Aldrich safer replacements for ethidium bromide. GelRed™ and GelGreen™ are non-toxic and (catalog no. 235474) also was used for reference. non-mutagenic because they do not bind DNA in living cells due to their membrane impermeability. -

Gelred™& Gelgreen™
GelRed™ & GelGreen™ Safe and sensitive nucleic acid gel stains elRed™ and GelGreen™ are next-generation fluorescent nucleic acid gel stains designed to replace the highly toxic ethidium bromide (EtBr). Developed by G scientists at Biotium, GelRed™ and GelGreen™ are superior to EtBr and other SYBR® Safe GelRed™ GelGreen™ EtBr alternatives by having a combination of low toxicity, high sensitivity and exceptional stability. EtBr has been the predominant dye used for nucleic acid gel staining for decades because of its low price and generally sufficient sensitivity. However, EtBr is a highly mutagenic chemical. The safety hazard and costs associated with decontamination and waste disposal can ultimately make the dye expensive and inconvenient to use. For this reason, alternative gel stains, such as SYBR® dyes, have become commercially available Figure 2. GelRed™ and GelGreen™ gel stains are safer because they cannot penetrate cell in recent years. While these alternative dyes have reduced mutagenicity, they sacrifice membranes to bind DNA in living cells. HeLa cells were incubated at 37oC with 1X SYBR® Safe, sensitivity and stability. For example, SYBR® Safe has very limited sensitivity while GelGreen™ or GelRed™, respectively. Images were taken following incubation with dye for 30 SYBR® Green and SYBR® Gold are much less stable than EtBr. SYBR® dyes also enter min using FITC filter set for SYBR® Safe and GelGreen™, and Cy®3 filter set for GelRed™. SYBR® Safe rapidly entered cells and stained nuclei. GelRed™ and GelGreen™ were unable cells rapidly to stain mitochondria and nuclear DNA, making it more likely for the dyes to to cross cell membranes, demonstrated by the absence of fluorescence staining. -

Tech Tip for Staining with Gelred and Gelgreen
Tech Tip: Eight things to keep in mind for optimal gel staining with GelRed® and GelGreen® The most common use of GelRed® and GelGreen® nucleic acid stains is in pre-cast agarose gels, where the dyes are added to molten agarose during gel preparation. However, due to their larger size designed to improve safety, band migration of DNA in pre-cast gels may be affected. Some samples, such as restriction digested DNA can migrate abnormally in GelRed or GelGreen precast gels. GelRed and GelGreen are larger dyes than ethidium bromide, a design feature related to their safety This is why overloading DNA results in smearing, smiling, or curvy bands, and aberrant DNA migration. The recommended loading amount for samples of known concentration or commercial ladders/ DNA markers is 50-200 ng/ lane. For samples of unknown concentration, loading 1/2 – 1/4 the usual amount of DNA usually solves migration issues. GelRed and GelGreen are ultra-sensitive dyes, more sensitive than ethidium bromide Low DNA amounts (0.1 ng or lower) can be reliably detected. Loading high DNA amounts is not required, and can result in issues with DNA migration. Loading lower DNA amounts gives better separation and sharper bands, saving on precious samples, DNA ladder and conserving costs. GelRed and GelGreen do not migrate through the gel as easily as ethidium bromide, giving more homogenous staining throughout the gel Loss of staining, or faint staining of low molecular weight DNA bands in not an issue as GelRed and GelGreen do not migrate as fast as ethidium bormide through the gel. -

Gelred™ Nucleic Acid Gel Stain – 10000X
Datasheet GelRED™ Nucleic Acid Gel Stain – 10000X Product description GelRed™ is an ultra-sensitive, extremely stable and environmentally safe fluorescent nucleic acid dye designed to replace the highly toxic ethidium bromide (EB) for staining dsDNA, ssDNA or RNA in agarose gels or polyacrylamide gels. GelRed is far more sensitive than EB without requiring a destaining step. GelRed and EB have virtually the same spectra, so you can directly replace EB with GelRed without changing your existing imaging system. GelRed™ can be used to stain dsDNA, ssDNA or RNA in agarose gel via either precast or post gel staining. GelRed can also be used to stain dsDNA, ssDNA or RNA in polyacrylamide gel via post gel staining. GelRed is also compatible with downstream DNA manipulations such as digestion with a restriction enzyme, Southern blotting techniques and cloning. A series of safety tests have confirmed that GelRed™ is noncytotoxic, nonmutagenic and nonhazardous at concentrations well above the working concentrations used in gel staining. As a result, GelRed can be safely disposed of in regular trash, providing convenience and reducing cost in waste disposal.GelRed™ is supplied as 10,000X solution in water or for your convenience. As nucleic acid binding dyes can affect DNA migration during electrophoresis, post-staining of gels is highly recommended. Post- staining with GelRed™ results in superior sensitivity and eliminates the possibility of dye interference with DNA migration. Post- staining with GelRed™ is simple, requiring no destaining and no special buffer. Simply dilute the concentrated dye in 0.1M NaCl or water and incubate the gel in the diluted dye solution for 30 minutes. -

A Comparison of DNA Stains and Staining Methods for Agarose Gel Electrophoresis
bioRxiv preprint doi: https://doi.org/10.1101/568253; this version posted March 6, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. A comparison of DNA stains and staining methods for Agarose Gel Electrophoresis Andie C. Hall, Core Research Laboratories. Natural History Museum, London SW75BD. [email protected] +442079425048 ORCID iD 0000-0001-5546-7255 ABSTRACT Nucleic acid stains are necessary for Agarose Gel Electrophoresis (AGE). The commonly used but mutagenic Ethidium Bromide is being usurped by a range of safer but more expensive alternatives. These safe stains vary in cost, sensitivity and the impedance of DNA as it migrates through the gel. Modified protocols developed to reduce cost increase this variability. In this study, five Gel stains (GelRed™, GelGreen™, SYBR™ safe, SafeView and EZ-Vision®In-Gel Solution) two premixed loading dyes (SafeWhite, EZ-Vision®One) and four methods (pre-loading at 100x, pre-loading at 10x, precasting and post-staining) are evaluated for sensitivity and effect on DNA migration. GelRed™ was found to be the most sensitive while the EZ-Vision® dyes and SafeWhite had no discernible effect on DNA migration. Homemade loading dyes were as effective as readymade ones at less than 4% of the price. This method used less than 1% of the dye needed for the manufacturer recommended protocols. Thus, with careful consideration of stain and method, Gel stain expenditure can be reduced by over 99%. -

Gelred® Product Information Sheet
Glowing Products for Science™ www.biotium.com Revised: January 15, 2021 Product Information GelRed® Nucleic Acid Gel Stain, 10,000X Considerations for Staining The following are general considerations for staining gels with GelRed®. See the Catalog no. Product Size staining protocols on page 2 for step by step directions. 41002 GelRed® 10,000X in DMSO 0.5 mL • GelRed® can be used as precast in agarose gels or post-staining protocols. Post-staining with GelRed® is recommended and results in superior 41002-1 GelRed® 10,000X in DMSO 10 mL sensitivity and eliminates the possibility of dye interference with DNA 41003-T GelRed® 10,000X in water 0.1 mL migration. 41003 GelRed® 10,000X in water 0.5 mL • The precast protocol is not recommended for acrylamide gels. For acrylamide gels we recommend post-staining. Biotium also offers PAGE 41003-1 GelRed® 10,000X in water 10 mL GelRed® which is especially formulated for staining the denser gel matrix in polyacrylamide gels. Storage and Handling • GelRed® can be used with any commonly used loading buffer in precast and GelRed® is a very stable dye. Store 10,000X solution and dilute solutions of post-stained GelRed® gels. We have had good results with 6X loading buffer GelRed® at room temperature, protected from light. Dye precipitation may occur containing 15% glycerol, 7.5% Ficoll® 400, 0.05% Bromophenol Blue. As at lower temperatures, resulting in lower signal or the appearance of precipitate on a tracking dye we have also used 0.1% Patent Blue VF or 0.1% Orange G the surface of the gel. -
Cupriavidus Metallidurans Strains with Different Mobilomes and from Distinct Environments Have Comparable Phenomes
G C A T T A C G G C A T genes Article Cupriavidus metallidurans Strains with Different Mobilomes and from Distinct Environments Have Comparable Phenomes Rob Van Houdt 1,* , Ann Provoost 1, Ado Van Assche 2, Natalie Leys 1 , Bart Lievens 2, Kristel Mijnendonckx 1 and Pieter Monsieurs 1 1 Microbiology Unit, Belgian Nuclear Research Centre (SCK•CEN), B-2400 Mol, Belgium; [email protected] (A.P.); [email protected] (N.L.); [email protected] (K.M.); [email protected] (P.M.) 2 Laboratory for Process Microbial Ecology and Bioinspirational Management, KU Leuven, B-2860 Sint-Katelijne-Waver, Belgium; [email protected] (A.V.A.); [email protected] (B.L.) * Correspondence: [email protected] Received: 21 September 2018; Accepted: 15 October 2018; Published: 18 October 2018 Abstract: Cupriavidus metallidurans has been mostly studied because of its resistance to numerous heavy metals and is increasingly being recovered from other environments not typified by metal contamination. They host a large and diverse mobile gene pool, next to their native megaplasmids. Here, we used comparative genomics and global metabolic comparison to assess the impact of the mobilome on growth capabilities, nutrient utilization, and sensitivity to chemicals of type strain CH34 and three isolates (NA1, NA4 and H1130). The latter were isolated from water sources aboard the International Space Station (NA1 and NA4) and from an invasive human infection (H1130). The mobilome was expanded as prophages were predicted in NA4 and H1130, and a genomic island putatively involved in abietane diterpenoids metabolism was identified in H1130. An active CRISPR-Cas system was identified in strain NA4, providing immunity to a plasmid that integrated in CH34 and NA1. -

Imaging Nucleic Acid Gels on Odyssey® Imagers
Application Guide Imaging Nucleic Acid Gels on Odyssey® Imagers Published July 2021. The most recent version of this document is posted at licor.com/bio/support. Page 2 - Imaging Nucleic Acid Gels on Odyssey® Imagers Table of Contents Page I. Background 2 II. Introduction 3 III. Stain Comparison 4 IV. Separation and Detection Protocol 5 Suggested Materials 5 In-Gel Prestaining 6 Post-Electrophoresis Staining 7 E-Gel® Pre-Cast Agarose Gels 8 V. Imaging Protocol 8 I. Background A common method for separating and analyzing nucleic acid molecules is nucleic acid gel electrophoresis. Nucleic acid gel electrophoresis separates nucleic acids based on molecular size and subsequent ability to move through an agarose gel. Depending on the experiment, a nucleic acid sample can be stained during or after separation. The gel is placed in the electrophoresis apparatus and buffer added to submerge the gel. Nucleic acid samples and size markers are loaded then separated on the gel using an electric field. The stained and separated nucleic acid molecules are visualized in the gel then compared to the band markers to estimate their sizes. There are numerous commercial stains that may be appropriate for imaging the results of nucleic acid electrophoresis. Common stains include Ethidium Bromide (EtBr), SYBR® Safe DNA stain, SYTO™ 60 Nucleic Acid Stain, and GelRed® Nucleic Acid Stain. Note: Always verify that a stain’s excitation and emission wavelengths are compatible with the intended instrument prior to use. Imaging Nucleic Acid Gels on Odyssey® Imagers - Page 3 II. Introduction This technical note explains the respective stain imaging capabilities of each Odyssey® Imager and the D-DiGit® Gel Scanner and how to effectively detect and image results.