PROGESTERONE INJECTION USP in SESAME OIL for INTRAMUSCULAR USE ONLY Rx Only
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

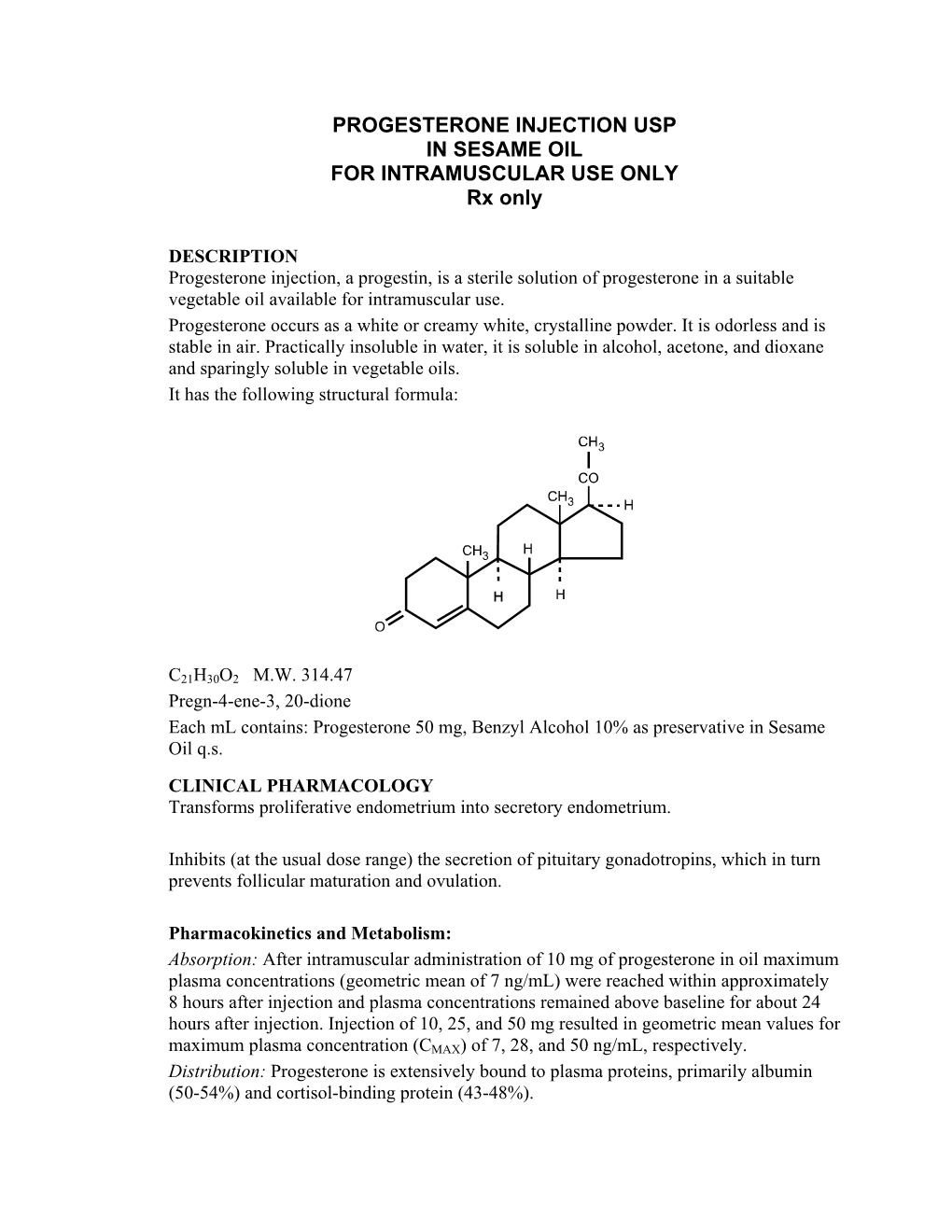
Salivary 17 Α-Hydroxyprogesterone Enzyme Immunoassay Kit
SALIVARY 17 α-HYDROXYPROGESTERONE ENZYME IMMUNOASSAY KIT For Research Use Only Not for use in Diagnostic Procedures Item No. 1-2602, (Single) 96-Well Kit; 1-2602-5, (5-Pack) 480 Wells Page | 1 TABLE OF CONTENTS Intended Use ................................................................................................. 3 Introduction ................................................................................................... 3 Test Principle ................................................................................................. 4 Safety Precautions ......................................................................................... 4 General Kit Use Advice .................................................................................... 5 Storage ......................................................................................................... 5 pH Indicator .................................................................................................. 5 Specimen Collection ....................................................................................... 6 Sample Handling and Preparation ................................................................... 6 Materials Supplied with Single Kit .................................................................... 7 Materials Needed But Not Supplied .................................................................. 8 Reagent Preparation ....................................................................................... 9 Procedure ................................................................................................... -

Cortisol Deficiency and Steroid Replacement Therapy
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Cortisol deficiency and steroid replacement therapy This leaflet explains about cortisol deficiency and how it is treated. It also contains information about how to deal with illnesses, accidents and other stressful events in children on cortisol replacement. Where are the The two most important ones are: adrenal glands and • Aldosterone – this helps regulate what do they do? the blood pressure by controlling how much salt is retained in the The adrenal glands rest on the tops body. If a person is unable to of the kidneys. They are part of the make aldosterone themselves, they endocrine system, which organises the will need to take a tablet called release of hormones within the body. ‘fludrocortisone’. Hormones are chemical messengers that switch on and off processes within the • Cortisol – this is the body’s natural body. steroid and has three main functions: The adrenal glands consist of two parts: - helping to control the blood the medulla (inner section) which sugar level makes the hormone ‘adrenaline’ which is part of the ‘fight or flight’ - helping the body deal with stress response a person has when stressed. - helping to control blood pressure the cortex (outer section) which and blood circulation. releases several hormones. If a person is unable to make cortisol themselves, they will need to take a tablet to replace it. Pituitary gland The most common form used is hydrocortisone, but other forms Parathyroid gland may be prescribed. Thyroid gland Medulla Cortex Adrenal Thymus gland Gland Kidney Adrenal gland Pancreas Sheet 1 of 7 Ref: 2014F0715 © GOSH NHS Foundation Trust March 2015 What is In these circumstances, the amount cortisol deficiency? of hydrocortisone given needs to be increased quickly. -

Systemic Effect of Inhaled Corticosteroids on Adrenal
Issa-El-Khoury et al. Allergy, Asthma & Clinical Immunology (2015) 11:9 DOI 10.1186/s13223-015-0075-z ALLERGY, ASTHMA & CLINICAL IMMUNOLOGY POSITION ARTICLE AND GUIDELINES Open Access CSACI position statement: systemic effect of inhaled corticosteroids on adrenal suppression in the management of pediatric asthma Karine Issa-El-Khoury1, Harold Kim2,3, Edmond S Chan4, Tim Vander Leek5 and Francisco Noya1* Abstract Asthma is a chronic inflammatory disease of the airways that affects a growing number of children and adolescents. Inhaled corticosteroids (ICS) are the mainstay of treatment in persistent asthma, with a stepwise approach to increasing doses of ICS depending on asthma severity and control. ICS have known local and systemic side effects, of which adrenal suppression is still under-recognized. The latter is associated with chronic exposure and higher doses, although it has rarely been reported in children receiving low doses for a short period of time. The Canadian Society of Allergy and Clinical Immunology (CSACI) therefore recommends that physicians screen for adrenal suppression in children receiving high doses for more than 6 months and to consider screening those on medium dose if the risk is deemed higher by factors that increase an individual’s systemic corticosteroid exposure. Morning serum cortisol level can be used as a screening tool and abnormal results or normal results with a high index of suspicion should be confirmed with low-dose ACTH stimulation tests. Keywords: Asthma, Inhaled corticosteroids, Fluticasone, Adrenal suppression Background reducing asthma mortality. Most patients can achieve Asthma affects about 10% of the Canadian population, and asthma control using relatively low doses of ICS, which 50-80% of children affected develop it before the age of 5 will produce maximum or near-maximum clinical benefit, years [1]. -

CORTISOL IMBALANCE Patient Handout
COMMON PATTERNS OF CORTISOL IMBALANCE Patient HandOut Cortisol that does not follow the normal pattern can trigger blood sugar imbalances, food cravings and fat storage, especially around the middle. Related imbalances of low DHEA commonly result in loss of lean muscle, lack of strength, decreased stamina and low exercise tolerance. Chronically Elevated Cortisol Overall higher than normal cortisol Lifestyle suggestions: production throughout the day from • Reduce stress and improve coping skills prolonged stress demands. High • Protein at each meal, no skipping lunch cortisol also depletes its precursor hormone progesterone. • Hydrate throughout the day, herbal teas and water, avoid soft drinks General symptoms: • Reduce consumption of refined carbohydrates and caffeine • Food/sugar cravings • Get adequate sleep (at least 7 hours); catnaps • Feeling “tired but wired” • Aerobic exercise: <40 min low – moderate intensity • Insomnia during time when cortisol level within optimal range • Anxiety • Strength training: with guidance 2-3 times per week • Enjoy exercise that decreases excessive stress symptoms Steep Drop in Cortisol • Exercise in the morning Stress/fatigued pattern – morning Lifestyle suggestions: cortisol in the high normal range or • Reduce stress and improve coping skills elevated, but levels drop off rapidly, • Protein at each meal, no skipping lunch indicating adrenal dysfunction. • Hydrate throughout the day, herbal teas General symptoms: and water, avoid soft drinks • Mid-day energy drop • Reduce consumption of refined carbohydrates and caffeine • Drowsiness • Get adequate sleep (at least 7 hours); catnaps • Caffeine/sugar cravings • Exercise mid morning to boost energy with a combination • Low exercise tolerance/ of muscle building and cardiovascular activities poor recovery • Schedule more time for fun activities Rebound Cortisol Up and down/ irregular cortisol, Lifestyle suggestions: not following the normal pattern. -

Sleep Deprivation on the Nighttime and Daytime Profile of Cortisol Levels
Sleep. 20(10):865-870 © 1997 American Sleep Disorders Association and Sleep Research Society Sleep Loss Sleep Loss Results in an Elevation of Cortisol Levels the Next Evening Downloaded from https://academic.oup.com/sleep/article/20/10/865/2725962 by guest on 30 September 2021 *Rachel Leproult, tGeorges Copinschi, *Orfeu Buxton and *Eve Van Cauter *Department of Medicine, University of Chicago, Chicago, Illinois, U.S.A.; and tCenter for the Study of Biological Rhythms and Laboratory of Experimental Medicine, Erasme Hospital, Universite Libre de Bruxelles, Brussels, Belgium Summary: Sleep curtailment constitutes an increasingly common condition in industrialized societies and is thought to affect mood and performance rather than physiological functions. There is no evidence for prolonged or delayed effects of sleep loss on the hypothalamo-pituitary-adrenal (HPA) axis. We evaluated the effects of acute partial or total sleep deprivation on the nighttime and daytime profile of cortisol levels. Plasma cortisol profiles were determined during a 32-hour period (from 1800 hours on day I until 0200 hours on day 3) in normal young men submitted to three different protocols: normal sleep schedule (2300-0700 hours), partial sleep deprivation (0400-0800 hours), and total sleep deprivation. Alterations in cortisol levels could only be demonstrated in the evening following the night of sleep deprivation. After normal sleep, plasma cortisol levels over the 1800-2300- hour period were similar on days I and 2. After partial and total sleep deprivation. plasma cortisol levels over the 1800-2300-hour period were higher on day 2 than on day I (37 and 45% increases, p = 0.03 and 0.003, respec tively), and the onset of the quiescent period of cortisol secretion was delayed by at least I hour. -

Quantification of the Hormones Progesterone and Cortisol in Whale
Quantification of the Hormones Progesterone and Cortisol in Whale Breath Samples Using Novel, Non-Invasive Sampling and Analysis with Highly-Sensitive ACQUITY UPLC and Xevo TQ-S Jody Dunstan,1 Antonietta Gledhill,1 Ailsa Hall,2 Patrick Miller,2 Christian Ramp3 1Waters Corporation, Manchester, UK 2Scottish Oceans Institute, University of St. Andrews, UK 3Mingan Island Cetacean Study, St Lambert, Quebec, Canada APPLICATION BENEFITS INTRODUCTION ■■ Provides a novel, non-invasive sampling method The conservation and management of large whale populations require information to obtain sample from whale blow. These about all aspects of their biology, life history, and behavior. However, it is samples can then be analyzed to determine extremely difficult to determine many of the important life history parameters, the levels of progesterone and cortisol. such as reproductive status, without using lethal or invasive methods. As such, efforts are now focused on obtaining as much information as possible from the ■■ A sensitive, repeatable, quantitative LC-tandem quadrupole MS method is shown. samples collected remotely, with a minimum of disturbance to the whales. Various excreted samples, such as sloughed skin and feces are being used to determine ■■ Parallel acquisition of MRM and full scan sex and maturity, as well as life history stage.1,2 MS data allows both the compounds of interest, and also the matrix background Attention has recently shifted more towards what can be analyzed from samples to be monitored. of whale blow for health assessment3 or steroid hormone analysis.4 Hormone analysis is of particular interest as high levels of progesterone can be used as an ■■ Simultaneous quantitative and investigative indicator of pregnancy status, while other steroids, such as glucocorticoids may be analysis can be performed for different markers of the short-term, acute stress response. -

Auspar Attachment 1: Product Information for Progesterone
Attachment 1: Product information for AusPAR - ORIPRO - Progesterone - Orion Laboratories Ltd (T/A Perrigo Australia) - PM-2018-04477-1-5 FINAL 7 February 2020. This is the Product Information that was approved with the submission described in this AusPAR. It may have been superseded. For the most recent PI, please refer to the TGA website at <https://www.tga.gov.au/product-information-pi> AUSTRALIAN PRODUCT INFORMATION – ORIPRO® (PROGESTERONE) PESSARIES 1 NAME OF THE MEDICINE Progesterone 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Oripro Pessaries contain as active substance 100mg or 200mg of progesterone (micronized) in hard fat. 3 PHARMACEUTICAL FORM Vaginal Pessaries - Opaque, bullet-shaped waxy, solid masses. 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Oripro Pessaries are indicated for: 1. Assisted reproductive technology (ART) treatment of infertile women with progesterone deficiency, requiring progesterone supplementation or replacement to support embryo implantation and maintain initial pregnancy. 2. Prevention of preterm birth in singleton pregnancies at risk due to; · Shortened cervix 1 Attachment 1: Product information for AusPAR - ORIPRO - Progesterone - Orion Laboratories Ltd (T/A Perrigo Australia) - PM-2018-04477-1-5 FINAL 7 February 2020. This is the Product Information that was approved with the submission described in this AusPAR. It may have been superseded. For the most recent PI, please refer to the TGA website at <https://www.tga.gov.au/product-information-pi> The dosage of progesterone for prevention of preterm birth is 200 mg daily (at night). Treatment can be initiated during the second trimester (16-24 weeks gestation) and is to be continued to the end of the 36th week of gestation or until delivery. -

Pharmacology/Therapeutics II Block III Lectures 2013-14
Pharmacology/Therapeutics II Block III Lectures 2013‐14 66. Hypothalamic/pituitary Hormones ‐ Rana 67. Estrogens and Progesterone I ‐ Rana 68. Estrogens and Progesterone II ‐ Rana 69. Androgens ‐ Rana 70. Thyroid/Anti‐Thyroid Drugs – Patel 71. Calcium Metabolism – Patel 72. Adrenocorticosterioids and Antagonists – Clipstone 73. Diabetes Drugs I – Clipstone 74. Diabetes Drugs II ‐ Clipstone Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones Date: Thursday, March 20, 2014-8:30 AM Reading Assignment: Katzung, Chapter 37 Key Concepts and Learning Objectives To review the physiology of neuroendocrine regulation To discuss the use neuroendocrine agents for the treatment of representative neuroendocrine disorders: growth hormone deficiency/excess, infertility, hyperprolactinemia Drugs discussed Growth Hormone Deficiency: . Recombinant hGH . Synthetic GHRH, Recombinant IGF-1 Growth Hormone Excess: . Somatostatin analogue . GH receptor antagonist . Dopamine receptor agonist Infertility and other endocrine related disorders: . Human menopausal and recombinant gonadotropins . GnRH agonists as activators . GnRH agonists as inhibitors . GnRH receptor antagonists Hyperprolactinemia: . Dopamine receptor agonists 1 Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. 1. Overview of Neuroendocrine Systems The neuroendocrine -

Progesterone – an Amazing Hormone Sheila Allison, MD
Progesterone – An Amazing Hormone Sheila Allison, MD Management of abnormal PAP smears and HPV is changing rapidly as new research information is available. This is often confusing for physicians and patients alike. I would like to explain and hopefully clarify this information. Almost all abnormal PAP smears and cervical cancers are caused by the HPV virus. This means that cervical cancer is a sexually transmitted cancer. HPV stands for Human Papilloma Virus. This is a virus that is sexually transmitted and that about 80% of sexually active women are exposed to. The only way to absolutely avoid exposure is to never be sexually active or only have intercourse with someone who has not had intercourse with anyone else. Because most women become sexually active in their late teens and early 20s, this is when most exposures occur. We do not have medication to eradicate viruses (when you have a cold, you treat the symptoms and wait for the virus to run its course). Most women will eliminate the virus if they have a healthy immune system and it is then of no consequence. There are over 100 subtypes of the HPV virus. Most are what we call low-risk viruses. These are associated with genital warts and are rarely responsible for abnormal cells and cancer. Two of these subtypes are included in the vaccine that is now recommended prior to initiating sexual activity. Few women who see me for hormone management will leave without a progesterone prescription. As a matter of fact, I have several patients who are not on any estrogen but are on progesterone exclusively. -

Australian Public Assessment Report for Progesterone
Australian Public Assessment Report for Progesterone Proprietary Product Name: Prometrium / Utrogestan Sponsor: Besins Healthcare Australia Pty Ltd June 2017 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance) when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website <https://www.tga.gov.au>. About AusPARs • An Australian Public Assessment Report (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. • AusPARs are prepared and published by the TGA. • An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations and extensions of indications. • An AusPAR is a static document; it provides information that relates to a submission at a particular point in time. • A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -

Mifepristone (Korlym)
Drug and Biologic Coverage Policy Effective Date ............................................ 1/1/2021 Next Review Date… ..................................... 1/1/2022 Coverage Policy Number ............................... IP0092 Mifepristone (Korlym®) Table of Contents Related Coverage Resources Overview .............................................................. 1 Coverage Policy ................................................... 1 Reauthorization Criteria ....................................... 2 Authorization Duration ......................................... 2 Conditions Not Covered....................................... 2 Background .......................................................... 3 References .......................................................... 4 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage -

Hydrocortisone Tablets Contain Lactose Monohydrate
New Zealand Data Sheet 1. PRODUCT NAME Hydrocortisone 5 mg Tablets Hydrocortisone 20 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each Hydrocortisone 5 mg Tablet contains 5 mg of hydrocortisone. Each Hydrocortisone 20 mg Tablet contains 20 mg of hydrocortisone. Excipient(s) with known effect Hydrocortisone Tablets contain lactose monohydrate. For the full list of excipients, see Section 6.1. 3. PHARMACEUTICAL FORM Hydrocortisone 5 mg Tablet: white, round, biconvex tablet having a diameter of 6.5 mm. Hydrocortisone 20 mg Tablet: white, round, biconvex tablet having a diameter of 7.94 mm, breakline on one face and dp logo on the other. The score line on Hydrocortisone 20 mg Tablet is only to facilitate breaking for ease of swallowing and not to divide into equal doses. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications • Replacement therapy in Addison’s disease or chronic adrenocortical insufficiency secondary to hypopituitarism. • Inhibition of the secondary increase in ACTH secretion when aminoglutethimide is administered for breast or prostatic cancer. 4.2. Dose and method of administration Dose As replacement therapy The normal requirement is 10‐30 mg daily (usually 20 mg in the morning and 10 mg at night to mimic the circadian rhythm of the body). 1 | Page As combination therapy with aminoglutethimide A dosage of 40 mg daily, given as 10 mg with breakfast, 10 mg with dinner and 20 mg at bedtime is usually recommended. Special populations Elderly population Steroids should be used cautiously in the elderly, since adverse effects are enhanced in old age, see Section 4.4. When long term treatment is to be discontinued, the dose should be gradually reduced over a period of weeks or months, depending on dosage and duration of therapy, see Section 4.4.