The Effects of Mango on Gut Microbial Population and Its
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 'Van Dyke' Mango
7. MofTet, M. L. 1973. Bacterial spot of stone fruit in Queensland. 12. Sherman, W. B., C. E. Yonce, W. R. Okie, and T. G. Beckman. Australian J. Biol. Sci. 26:171-179. 1989. Paradoxes surrounding our understanding of plum leaf scald. 8. Sherman, W. B. and P. M. Lyrene. 1985. Progress in low-chill plum Fruit Var. J. 43:147-151. breeding. Proc. Fla. State Hort. Soc. 98:164-165. 13. Topp, B. L. and W. B. Sherman. 1989. Location influences on fruit 9. Sherman, W. B. and J. Rodriquez-Alcazar. 1987. Breeding of low- traits of low-chill peaches in Australia. Proc. Fla. State Hort. Soc. chill peach and nectarine for mild winters. HortScience 22:1233- 102:195-199. 1236. 14. Topp, B. L. and W. B. Sherman. 1989. The relationship between 10. Sherman, W. B. and R. H. Sharpe. 1970. Breeding plums in Florida. temperature and bloom-to-ripening period in low-chill peach. Fruit Fruit Var. Hort. Dig. 24:3-4. Var.J. 43:155-158. 11. Sherman, W. B. and B. L. Topp. 1990. Peaches do it with chill units. Fruit South 10(3): 15-16. Proc. Fla. State Hort. Soc. 103:298-299. 1990. THE 'VAN DYKE' MANGO Carl W. Campbell History University of Florida, I FAS Tropical Research and Education Center The earliest records we were able to find on the 'Van Homestead, FL 33031 Dyke' mango were in the files of the Variety Committee of the Florida Mango Forum. They contain the original de scription form, quality evaluations dated June and July, Craig A. -
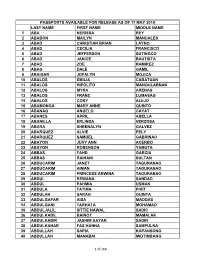
Passports Available for Release As of 17 May 2019
PASSPORTS AVAILABLE FOR RELEASE AS OF 17 MAY 2019 LAST NAME FIRST NAME MIDDLE NAME 1 ABA NERISSA REY 2 ABABON MAILYN MANUALES 3 ABAD CHRISTIAN BRIAN LAYNO 4 ABAD CECILIA FRANCISCO 5 ABAD JEFFERSON SUTINGCO 6 ABAD JANICE BAUTISTA 7 ABAD ZOE RAMIREZ 8 ABAG DALE GAMIL 9 ABAIGAR JOFALYN MOJICA 10 ABALOS EMILIA CABATUAN 11 ABALOS HIPOLITO MANGULABNAN 12 ABALOS MYRA ARENAS 13 ABALOS FRANZ LUMASAG 14 ABALOS CORY ALEJO 15 ABAMONGA MARY ANNE QUINTO 16 ABANAG ANGELO CAYAT 17 ABANES APRIL ABELLA 18 ABANILLA ERLINDA VERZOSA 19 ABARA SHEENALYN GALVEZ 20 ABARQUEZ ALVIE PELY 21 ABARQUEZ SAMUEL GABRINAO 22 ABAYON JUVY ANN ACERBO 23 ABAYON ROBENSON YAMUTA 24 ABBAS FAHD GARCIA 25 ABBAS RAIHANI SULTAN 26 ABDUCARIM JANET TAGURANAO 27 ABDUCARIM AIMAN TAGURANAO 28 ABDUCARIM PRINCESS ARWINA TAGURANAO 29 ABDUL REWANA SANDAD 30 ABDUL PAHMIA USMAN 31 ABDULA FATIMA PIKIT 32 ABDULAH SARAH GUINTA 33 ABDULGAFAR AISA MADDAS 34 ABDULGANI TARHATA MOHAMAD 35 ABDULJALIL SITTIE NAWAL SADIC 36 ABDULKADIL BAINOT MAMALAK 37 ABDULKADIR JASHIM AAYAN SADIN 38 ABDULKAHAR FAS HANNA SAMPULNA 39 ABDULLAH SAPIA KAPANSONG 40 ABDULLAH MANABAI MIDTIMBANG 1 of 166 PASSPORTS AVAILABLE FOR RELEASE AS OF 17 MAY 2019 LAST NAME FIRST NAME MIDDLE NAME 41 ABDULLAH NORHATA MANTLI 42 ABDULLAH RUGAYA KAMSA 43 ABDULLAH EDRIS SAMO 44 ABDULLAH MOHD RASUL II WINGO 45 ABDULRAKIM HANIFA BURANTONG 46 ABDURASAD RIRDALIN MAGARIB 47 ABEJERO MARICAR TADIFA 48 ABELARDO DODIE DEL ROSARIO 49 ABELGAS JUMIL TABORADA 50 ABELLA ERIC RITCHIE REX SY 51 ABELLA MARIVIC SILVESTRE 52 ABELLA JERREMY JACOB MONIS 53 ABELLA MARISSA CANILAO 54 ABELLA MELISSA LUCINA 55 ABELLANA RENANTE BUSTAMANTE 56 ABELLANIDA ROSE ANN GAGNO 57 ABELLANO JOHN PAUL ERIA 58 ABELLANOSA JOEL GALINDO 59 ABELLAR MA. -

Caribbean Sweet Potato Cups with Poached Eggs & Mango Hollandaise
Caribbean Sweet Potato Cups with Poached Eggs & Mango Hollandaise 2006 Sweet Rewards Overall Grand Prize Winner Ingredients: Mango Hollandaise 2 (15-ounce) cans Louisiana cut yams, drained 3 Egg yolks 1/2 Cup orange juice 1 Tablespoon lime juice 2 Tablespoons brown sugar 1 Teaspoon LA hot sauce 1/8 Teaspoon cayenne pepper 1/2 Cup butter, melted 1/2 Teaspoon salt 1 Mango, purred 2 Egg whites, lightly beaten 1/4 Teaspoon salt 1/3 Cup diced roasted red peppers 1 Tablespoon finely chopped chives 6 Whole eggs, poached Mango Hollandaise (recipe follows) 2 Tablespoons finely chopped cilantro (optional) Instructions: Caribbean Sweet Potato Cups Preheat oven to 425F. Spray a 6-cup muffin tin with cooking spray. In a medium- sized bowl, combine yams, orange juice, brown sugar, cayenne pepper, salt, and egg whites. Mash until smooth. Stir in the roasted red peppers and chives. Spoon the potato mixture into the prepared muffin tin. Bake for 10 to 12 minutes, or until lightly browned. Carefully remove sweet potato cups from muffin tin, and arrange each one on a serving plate. Top each with a poached egg, and spoon a generous portion of mango hollandaise over. Garnish with chopped cilantro, if desired.1 Mango Hollandaise In a blender, combine egg yolks, lime juice, and hot sauce. Blend for 30 seconds. With the blender still running, pour the melted butter in slowly, and continue to blend until emulsified. Fold in the pureed mango and the salt. Serving Sive: 6 Cook Time: 30 minutes Categories: Sweet Rewards Recipes Breakfast Recipes Credits: Veronica Callaghan Glastonbury, CT . -

COMPASSION a Festival of Musical Passions JUNE 5–15 GREAT ARCHETYPAL STORIES of SUFFERING, EMPATHY, and HOPE
COMPASSION A FESTIVAL OF MUSICAL PASSIONS JUNE 5–15 GREAT ARCHETYPAL STORIES OF SUFFERING, EMPATHY, AND HOPE CONSPIRARE.ORG 1 COMPASSION Diversify your Assets: FESTIVAL Invest in the Arts. PIETÀ JUNE 5-7, FREDERICKSBURG & AUSTIN CONSIDERING MATTHEW SHEPARD DURUFLÉ – REQUIEM JUNE 8, AUSTIN A GNOSTIC PASSION JUNE 10, AUSTIN J.S. BACH – ST. MATTHEW PASSION JUNE 14-15, AUSTIN We applaud the artists and patrons who invest in our community. CRAIG HELLA JOHNSON Artistic Director & Conductor ROBERT KYR & JOHN MUEHLEISEN Composers & Speakers SEASON SUSTAINING UNDERWRITER tm 2 3 TABLE OF CONTENTS FROM THE ARTISTIC DIRECTOR: WELCOME AND INTRODUCTION WELCOME AND INTRODUCTION .................................................................. 5-6 PROGRAM: PIETÀ ...............................................................................................7 ARTISTS: PIETÀ ..................................................................................................8 PROGRAM NOTES: PIETÀ ............................................................................ 9-10 PROGRAM: CONSIDERING MATTHEW SHEPARD/REQUIEM .......................... 11 Welcome to the Conspirare comPassion Festival. ARTISTS: CONSIDERING MATTHEW SHEPARD/REQUIEM .............................. 12 Whether you find yourself in the middle of a PROGRAM NOTES: CONSIDERING MATTHEW SHEPARD/REQUIEM .............. 13 performance or at a workshop, I invite you to PROGRAM: A GNOSTIC PASSION.................................................................... 14 take this time to deeply experience -
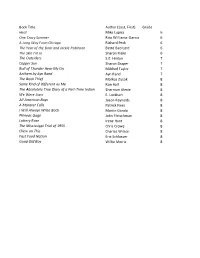
Book List 2020-2021
Book Title Author (Last, First) Grade Heat Mike Lupica 6 One Crazy Summer Rita Williams-Garcia 6 A Long Way From Chicago Richard Peck 6 The Year of the Boar and Jackie Robinson Bette Bao Lord 6 The Skin I’m In Sharon Flake 6 The Outsiders S.E. Hinton 7 Copper Sun Sharon Draper 7 Roll of Thunder Hear My Cry Mildred Taylor 7 Anthem by Ayn Rand Ayn Rand 7 The Book Thief Markus Zusak 8 Same Kind of Different as Me Ron Hall 8 The Absolutely True Diary of a Part-Time Indian Sherman Alexie 8 We Were Liars E. Lockhart 8 All American Boys Jason Reynolds 8 A Monster Calls Patrick Ness 8 I Will Always Write Back Martin Ganda 8 Phineas Gage John Fleischman 8 Lottery Rose Irene Hunt 8 The Mississippi Trial of 1955 Chris Crowe 8 Chew on This Charles Wilson 8 Fast Food Nation Eric Schlosser 8 Good Old Boy Willie Morris 8 Book Title Grade Author (Last, first) How They Croaked 8 Bragg, Georgia A Christmas Carol 8 Dickens, Charles I Am the Cheese 8 Cormier, Robert Panic 8 Draper, Sharon Copper Sun 8 Draper, Sharon And Then There Were None 8 Christie, Agatha I Will Always Write Back 8 Alifirenka, Caitlin; Ganda, Martin; Welch, Liz Three Little Words 8 Rhodes-Courter, Ashley Chew on This 8 Schlosser, Eric; Wilson, Charles Fast Food Nation 8 Schlosser, Eric A Monster Calls Accelerated Only 8 Ness, Patrick The President Has Been Shot! The Assassination of JKF. Accelerated Only 8 Swanson, James; Patton, Will Wintergirls 8 Anderson, Laurie Halse I Will Save You 8 De La Pena, Matt Three Weeks with My Brother Accelerated Only 8 Sparks, Nicholas; Sparks, Micah Chains 8 Anderson, Laurie Halse Freak the Mighty 7 Philbrick, Rodman The Naturals 7 Barnes, Jennifer Lynn Fearless 7 Michaels, Fern The Glory Fields 7 Myers, Walter Dean Countdown 7 Wiles, Deborah Three Little Words Acclerated Only 7 Rhodes-Courter, Ashley Counting By 7's 7 Sloan, Holly Goldberg And Then There Were None Acclerated Only 7 Christie, Agatha The Outsiders 7 Hinton, S.E. -

DURING FROZEN STORAGE by ANGELA RINCON
EFFECT OF PREFREEZING TREATMENTS ON QUALITY OF MANGO (Manguifera Indica L.) DURING FROZEN STORAGE by ANGELA RINCON (Under the direction of William L. Kerr) ABSTRACT At the moment there is a high demand for high-quality fruit ingredients to be used in many food formulations such as pastry and confectionery products, ice cream, frozen desserts and sweets, fruit salads, cheese and yoghurt. The overall objective was to improve quality of frozen mango using two types of food cryoprotection: one was the reduction of water content of the fruit (osmotic dehydration) and the other was the formulation of mango pulp with carbohydrates of different molecular weight to increase frozen stability. Osmotic dehydration was able to modify quality parameters of slices before and after frozen storage. Values for some parameters such as vitamin C, lightness (L*), chroma (C*), and firmness for non osmotically dehydrated slices were significantly higher than osmotically dehydrated slices. However, treatments carried out with osmotic solutions (especially those with high concentrations of sucrose) improved significantly the quality of mango slices after frozen storage. Higher moisture losses and solid gain values were reported for slices from the highest osmotic solution concentration. Thus, slices dipped in 30oBrix were better protected against freezing damage. Effects of sucrose concentration on the slices and ripening stage on frozen-thawed mango flavor perception were determined. Six flavor descriptors (color, flavor, sweetness, sourness, firmness and juiciness) were evaluated by a sensory trained panel. All descriptors were affected by sugar content and ripening stage. To study the effect of mango fruit composition on frozen stability, five pulp samples were prepared and evaluated in terms of glass transition temperature modification and its influence on ascorbic acid retention. -

Basmati Rice Salad with Mango & Cucumber
GUASTELLO’S VILLAGE MARKET Basmati Rice Salad with Mango & Cucumber Ingredients: 2 ½ cups basmati rice Kosher salt ½ cup plus 1 tbl. extra virgin olive oil, more as needed 1/3 cup rice vinegar 1 tbl. Asian sesame oil 2 tbl. finely grated fresh ginger Freshly ground black pepper 1 ¼ cups diced mango (1/2-inch dice) 1 ¼ cups diced peeled cucumber (1/2-inch dice) ½ cup thinly sliced scallions ¼ cup chopped fresh cilantro leaves Directions: Rinse the rice under cold water and drain. Bring 7 cups of water to a boil in a 4-quart pot over high heat. Add 3/4 tsp. salt. Add the rice, reduce the heat to a simmer, and cook uncovered, stirring occasionally and adding more boiling water as necessary to keep the rice covered, until tender, about 10 to 15 minutes. Drain and rinse the rice with cold water to stop the cooking. Transfer the rice to a foil-lined rimmed baking sheet, drizzle with 1 Tbs. of the oil, and toss lightly to coat. Spread the rice on the baking sheet and cool completely at room temperature or in the refrigerator. Put the vinegar in a small bowl and gradually whisk in the remaining 1/2 cup of olive oil. Whisk in the sesame oil and ginger. Taste and season with salt, pepper, and additional vinegar or olive oils needed. Put the cooked and cooled rice in a large serving bowl and toss to break up any clumps. Add the mango, cucumber, scallions, cilantro, and 1/2 cup vinaigrette and toss. Taste and season as needed with more vinaigrette, salt, pepper, and serve. -

Development Initiatives on Mango Quality and Marketing in Thailand
Development initiatives on mango quality and marketing in Thailand Overview Variety and Region of growing Production Process Postharvest Process Marketing and Branding Quality assurance measures 2 Variety and Region of growing 305,000 ha of mangoes in 2008. 2.3 million tones of fruit. 12, 22, 9 and 9 million dollar for fresh, canned, dried and frozen mango respectively. Nam Dok Mai No.4, Nam Dok Mai Si Thong and Mahachanok are the main variety. 3 The main production area is spread throughout the country. Linking area approach are used to make a production plan starting from central, northeastern, northern and southern respectively. 4 Most of the fruit is sold in the main domestic markets but about 2% is exported overseas. Mainly to Singapore, Malaysia, China, Japan, South Korea and EU. 5 Commercial variety 6 7 8 9 Production Process 10 Central region Bed system Ridge-and-furrow configuration 11 Northern region Nearby the foot-hill zone 12 Northern region Highland areas 13 Northeastern region Rain shadow 14 Dwarf tree 15 16 17 Tip pruning 18 19 Synchronization Allows all of the stems in the canopy to be in the same physiological stage of maturity. Tip pruning causes a uniform flush of growth throughout the canopy. Removes growth – and flower – inhibiting factors in stems derived from the previous season’s flowering and fruiting panicles. Tip pruning also stimulates lateral shoot development forming 5 to 10 times the original number of productive stems. 20 Considerations for the flowering program Harvest of the previous season’s crop Timing of the dry and wet seasons High susceptibility of flowers and fruit to disease during rainy seasons 21 Thinning 22 23 Allows each remaining fruit to develop to its maximum size. -

Mangifera Indica (Mango)
PHCOG REV. REVIEW ARTICLE Mangifera Indica (Mango) Shah K. A., Patel M. B., Patel R. J., Parmar P. K. Department of Pharmacognosy, K. B. Raval College of Pharmacy, Shertha – 382 324, Gandhinagar, Gujarat, India Submitted: 18-01-10 Revised: 06-02-10 Published: 10-07-10 ABSTRACT Mangifera indica, commonly used herb in ayurvedic medicine. Although review articles on this plant are already published, but this review article is presented to compile all the updated information on its phytochemical and pharmacological activities, which were performed widely by different methods. Studies indicate mango possesses antidiabetic, anti-oxidant, anti-viral, cardiotonic, hypotensive, anti-infl ammatory properties. Various effects like antibacterial, anti fungal, anthelmintic, anti parasitic, anti tumor, anti HIV, antibone resorption, antispasmodic, antipyretic, antidiarrhoeal, antiallergic, immunomodulation, hypolipidemic, anti microbial, hepatoprotective, gastroprotective have also been studied. These studies are very encouraging and indicate this herb should be studied more extensively to confi rm these results and reveal other potential therapeutic effects. Clinical trials using mango for a variety of conditions should also be conducted. Key words: Mangifera indica, mangiferin, pharmacological activities, phytochemistry INTRODUCTION Ripe mango fruit is considered to be invigorating and freshening. The juice is restorative tonic and used in heat stroke. The seeds Mangifera indica (MI), also known as mango, aam, it has been an are used in asthma and as an astringent. Fumes from the burning important herb in the Ayurvedic and indigenous medical systems leaves are inhaled for relief from hiccups and affections of for over 4000 years. Mangoes belong to genus Mangifera which the throat. The bark is astringent, it is used in diphtheria and consists of about 30 species of tropical fruiting trees in the rheumatism, and it is believed to possess a tonic action on mucus fl owering plant family Anacardiaceae. -

Mangifera Indica CV. 'Nam Dok Mai Si Thong'
E3S Web of Conferences 187, 04006 (2020) https://doi.org/10.1051/e3sconf /202018704006 TSAE 2020 Overall precision test of near infrared spectroscopy on mango fruits (Mangifera indica CV. ‘Nam Dok Mai Si Thong’) by on-line and off-line systems Wachiraya Lekhawattana1*, and Panmanas Sirisomboon2 1King Mongkut’s Institute of Technology Ladkrabang, Department of Agricultural Engineering, Faculty of Engineering, Bangkok, Thailand. 2King Mongkut’s Institute of Technology Ladkrabang, NIR Spectroscopy Research Center for Agricultural Products and Foods, Department of Agricultural Engineering, Faculty of Engineering, Bangkok, Thailand. Abstract. The near infrared (NIR) spectroscopy both on-line and off-line scanning was applied on mango fruits (Mangifera indica CV. ‘Nam dok mai- si Thong’) for the overall precision test. The reference parameter was total soluble solids content (Brix value). The results showed that the off-line scanning had a higher accuracy than on-line scanning. The scanning repeatability of the off-line and on-line systems were 0.00199 and 0.00993, respectively. The scanning reproducibility of the off-line and online systems were 0.00279 and 0.00513, respectively. The reference of measurement ͦ repeatability was 0.2. The maximum coefficient of determination (R ΛΏΦ) of the reference measurement was 0.894. 1 Introduction Mango is an important economic crop, which can generate income for farmers in Thailand because of consumption demand in both domestic and international markets. According to the statistics of mangoes exports in 2017, The total production throughout was 2.017 million tons [2]. The most exported mango species include nam dok mai, Khiao Sawiei, Hnuk Klangwan, Chok Anan, Rad and Aok Rong, respectively. -

Mushroom Bowl 12 Black Barley, Sweet Potato, Chimichuri Couscous
BROOKLYN BREADS GRAB & GO SANDWICHES Brooklyn Bagel 4 Chicken Salad Sandwich 12 choice of: plain or everything hand pulled organic chicken salad, choice of: avocado smash, salmon lettuce, tomato, citrus aioli, cream cheese, regular cream cheese brioche bun or Vermont butter Ham & Gruyere Sandwich 12 SALAD & GRAINS grainy mustard on baguette Mushroom Bowl 12 Brie & Apple Sandwich 12 black barley, sweet potato, chimichuri apple brandy jam on baguette Couscous Salad 12 Lorem ipsum saron couscous, roasted peppers, Ricotta Tartine 12 cauliower, charred lemon ricotta cheese, sun dried tomato, Calabrian chilli honey, sea salt, on sour dough toast Sweets Pastries 4/6 T I B E E R A E S M O H E V G S O S Fresh Squeezed Juice 6 Bushwick Tea 4 Heart Healthy 12 choice of: orange or choice of: brooklyn breakfast, raspberry, blueberry, strawberry, grapefruit king earl, moroccan mint, acai juice, pomegranate juice k-town, carroll gardens or Harney & Sons Juice 4 ginger turmeric Protein Junkie 12 choice of: organic cranberry or peanut butter, banana, cocoa chips, organic apple Bushwick Iced Tea 4 salted honey, almond milk Mist Juice 9 Runa Iced Tea 4 Energizer 12 Choice of: Pear to the People, O Beat, kale, pineapple, banana, mango 24 Carrot Marquis Energy 4 choice of: superberry, citrus Detox 12 Jus by Julie Booster Shot 5 or mango ginger matcha, mango, banana, almond milk, spinach Q Drinks 4 Pilot Kombucha 7 choice of: cola, ginger beer, choice of: grapefruit mint, tonic, soda or grapefruit orange turmeric or pomegranate rose Just Water 4 D E C N C O E V O I Ò F F E Drip – 3/4 Cappuccino – 5 Turmeric Latte – 5 Espresso – 3.5 Latte – 5 Matcha Latte – 5 Macchiato – 4 Americano – 4 Cold Brew – 6 Cortado – 4.5 Chai Latte – 5 Iced Coee – 4. -

Evaluation of Mango (Mangifera Indical.) Cultivars for Flowering
International Journal of Bio-resource and Stress Management 2017, 8(4):505-509 Full Research Doi: HTTPS://DOI.ORG/10.23910/IJBSM/2017.8.4.1811a Evaluation of Mango (Mangifera indica L.) Cultivars for Flowering, Fruiting and Yield Attributes T. S. Hada*and A. K. Singh Dept. of Horticulture, Institute of Agricultural Sciences, Banaras Hindu University Varanasi, Uttar Pradesh (221 005), India Corresponding Author Article History T. S. Hada Article ID: AR1811a e-mail: [email protected] Received in 3rd May, 2017 Received in revised form 29th June, 2017 Accepted in final form 4th August, 2017 Abstract A field experiment was conducted to investigate the flowering, fruiting and yield attributes of some mango cultivars for the years 2014−15 and 2015−16. Outcome of the present research work revealed that the date of panicle emergence in different mango cultivars varied in both the years. It was observed that the panicle emergence was earliest in Gulabkhas and late in Fazli in both the years. Chausa produced maximum panicle length (35.19 cm) and breadth (24.76 cm). In the year 2014−15, earliest flowering was noted in Gulabkhas (Feb 17−Feb 26) and in the year 2015−16, earliest flowering was observed in Bombai (Feb 20−Feb 28) while, flowering was very late in Sepiya for both the years. Maximum number of flowers was observed in Langra (1839.13) while, minimum number of flowers was recorded in Amrapali (954.75). The duration of flowering varied from 22.50−36.00 days in different mango cultivars. Maximum value in terms of days was observed with Sepiya (39 days) while it was minimum (22.50 days) in case of Alphonso.