Cation-Exchange Capacity of Soils (Ammonium Acetate)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ammonia As a Refrigerant
1791 Tullie Circle, NE. Atlanta, Georgia 30329-2305, USA www.ashrae.org ASHRAE Position Document on Ammonia as a Refrigerant Approved by ASHRAE Board of Directors February 1, 2017 Expires February 1, 2020 ASHRAE S H A P I N G T O M O R R O W ’ S B U I L T E N V I R O N M E N T T O D A Y © 2017 ASHRAE (www.ashrae.org). For personal use only. Additional reproduction, distribution, or transmission in either print or digital form is not permitted without ASHRAE’s prior written permission. COMMITTEE ROSTER The ASHRAE Position Document on “Ammonia as a Refrigerant” was developed by the Society’s Refrigeration Committee. Position Document Committee formed on January 8, 2016 with Dave Rule as its chair. Dave Rule, Chair Georgi Kazachki IIAR Dayton Phoenix Group Alexandria, VA, USA Dayton, OH, USA Ray Cole Richard Royal Axiom Engineers, Inc. Walmart Monterey, CA, USA Bentonville, Arkansas, USA Dan Dettmers Greg Scrivener IRC, University of Wisconsin Cold Dynamics Madison, WI, USA Meadow Lake, SK, Canada Derek Hamilton Azane Inc. San Francisco, CA, USA Other contributors: M. Kent Anderson Caleb Nelson Consultant Azane, Inc. Bethesda, MD, USA Missoula, MT, USA Cognizant Committees The chairperson of Refrigerant Committee also served as ex-officio members: Karim Amrane REF Committee AHRI Bethesda, MD, USA i © 2017 ASHRAE (www.ashrae.org). For personal use only. Additional reproduction, distribution, or transmission in either print or digital form is not permitted without ASHRAE’s prior written permission. HISTORY of REVISION / REAFFIRMATION / WITHDRAWAL -

Phosphate-Based Treatments for Conservation of Stone
RILEM Technical Letters (2017) 2: 14‐19 DOI: http://dx.doi.org/10.21809/rilemtechlett.2017.34 Phosphate‐based treatments for conservation of stone Enrico Sassoni a* a Department of Civil, Chemical, Environmental and Materials Engineering, University of Bologna, Via Terracini 28, 40131, Bologna, Italy Received: 30 May 2017 / Accepted: 09 August 2017 / Published online: 9 October 2017 © The Author(s) 2017. This article is published with open access and licensed under a Creative Commons Attribution 4.0 International License. Abstract To overcome the limitations of currently available protectives and consolidants for carbonate stones (such as marble and limestone), in 2011 the use of calcium phosphate was proposed. The idea is forming calcium phosphates (ideally hydroxyapatite) as the reaction product between the substrate and an aqueous solution of a phosphate salt that the stone is treated with. In this paper, the studies aimed at identifying the best treatment conditions (in terms of nature and concentration of the phosphate precursor, solution pH, reaction time, ionic and organic additions) are first briefly summarized. Then, the efficacy of the phosphate treatment in protecting marble from dissolution in rain and restoring cohesion of weathered marble and limestone is discussed. Some recent studies on the use of the phosphate treatment on alternative substrates and some future steps for research on the topic are finally outlined. Keywords: Cultural heritage; Marble; Hydroxyapatite; Protection; Consolidation 1 Introduction improve mechanical properties, by providing a binding action between the stone grains. Organic products are A great part of cultural heritage objects (e.g. monuments, effective in improving mechanical properties, but again architectural decorations and statues) is made of carbonate they lack compatibility and durability. -

Preparing to Manufacture Hydrogen Peroxide
PREPARING TO MANUFACTURE HYDROGEN PEROXIDE Part of the Hydrogen Peroxide Propulsion Guide The early laboratory preparation of hydrogen peroxide was based on the technique that Thenard used during the initial preparation of hydrogen peroxide. In this technique, barium nitrate, purified by recrystallization, was decomposed by heating in air in a porcelain retort. The resulting oxide was further oxidized by heating in a stream of oxygen to a dull red heat. The barium peroxide which formed was then dampened, ground, and dissolved in hydrochloric acid (nitric acid was used in Thenard’s initial experiments). A slight excess of sulfuric acid was then added to precipitate barium sulfate and regenerate hydrochloric acid. The procedure of barium peroxide solution and sulfate precipitation was repeated several times in the same solution to increase the peroxide concentration (concentrations of up to 33 percent by weight hydrogen peroxide could be achieved in this manner). The concentrated solution containing water, hydrogen peroxide, and hydrochloric acid, along with accumulated impurities, was cooled with ice and saturated with barium peroxide; iron and manganese impurities in the solution were then precipitated out as phosphates. The hydrochloric acid was removed by the addition of silver sulfate and the sulfate ion was removed by the subsequent addition of barium oxide. Further concentration was accomplished by vacuum distillation until “no further density increase occurs.” Thenard reported that 100 w/o hydrogen peroxide (on the basis of density data and the measurement of the volume of oxygen released) could be obtained by this technique. The first record of commercial production of hydrogen peroxide appeared in the 1865 to 1875 period. -

Ammonia in Drinking-Water
WHO/SDE/WSH/03.04/01 English only Ammonia in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality _______________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1996. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use. -

Chemistry Inventory; Fall
CHEMISTRY FALL 2005 MSDS Mfg.'s Name Chemical Name Quantity Stored Storage Conditions (on file = 9) Aluminum 9 1.5 kg Aluminum chloride, anhydrous, 98.5% 9 0.2 kg Aluminum chloride · 6H2O 9 0.5 kg Aluminum hydroxide 9 0.5 kg Aluminum nitrate 9 0.5 kg Aluminum sulfate 9 0.5 kg Ammonia, concentrated 9 4.0 L Ammonium acetate 9 0.2 kg Ammonium chloride 9 Ammonium dihydrogen phosphate (monobasic) 9 0.4 kg J.T. Baker Ammonium hydrogen phosphate (dibasic) No 0.5 kg Ammonium nitrate 9 2.5 kg Ammonium oxalate 9 0.7 kg Ammonium peroxydisulfate 9 0.5 kg Ammonium sulfate 9 0.2 kg Antimony 9 0.4 kg Barium chloride, anhydrous 9 2.5 kg Barium chloride · 2H2O 9 2.5 kg Barium nitrate 9 0.8 kg Bismuth 9 2.0 kg Boric Acid 9 0.4 kg Brass 9 Bromine 9 2.5 kg Cadmium 9 0.1 kg Cadmium nitrate 9 0.3 kg Calcium acetate · xH2O 9 0.5 kg Calcium carbide 9 1.0 kg Calcium carbonate 9 2.2 kg Calcium chloride 9 1.0 kg Calcium hydroxide 9 0.3 kg Calcium nitrate · 4H2O 9 1.0 kg Calcium oxide 9 0.3 kg Calcium sulfate · 2H2O 9 1.0 kg Carbon 9 0.1 kg Ceric ammonium nitrate 9 0.5 kg Cesium chloride 9 0.01 kg Chromium 9 0.01 kg Chromium chloride 9 0.5 kg Chromium nitrate 9 0.5 kg Cobalt 9 0.025 kg Cobalt chloride 9 0.7 kg Cobalt nitrate 9 0.6 kg Copper (assorted) 9 4.0 kg Copper acetate 9 0.05 kg Copper chloride 9 0.1 kg Copper nitrate 9 3.5 kg Copper oxide 9 0.4 kg Cupric sulfate, anhydrous 9 0.5 kg Cupric sulfate · 5H2O 9 2.75 kg EDTA 9 0.6 kg Iodine 9 2.0 kg Iron (assorted) 9 5.0 kg MSDS Mfg.'s Name Chemical Name Quantity Stored Storage Conditions (on file = 9) Ferric ammonium -
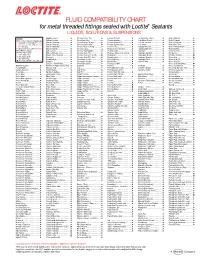
FLUID COMPATIBILITY CHART for Metal Threaded Fittings Sealed with Loctite¨ Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS
FLUID COMPATIBILITY CHART for metal threaded fittings sealed with Loctite® Sealants LIQUIDS, SOLUTIONS & SUSPENSIONS LEGEND: Bagasse Fibers.......................... Chlorobenzene Dry ................... Ferrous Chloride ...................... Ion Exclusion Glycol ................. Nickel Chloride.......................... All Loctite® Anaerobic Sealants are Barium Acetate ........................ Chloroform Dry......................... Ferrous Oxalate......................... Irish Moss Slurry...................... Nickel Cyanide ......................... Compatible Including #242®, 243, Barium Carbonate..................... Chloroformate Methyl............... Ferrous Sulfate10%.................. Iron Ore Taconite ..................... Nickel Fluoborate ..................... 542, 545, 565, 567, 569, 571, 572, Barium Chloride........................ Chlorosulfonic Acid .................. Ferrous Sulfate (Sat)................. Iron Oxide ................................ Nickel Ore Fines ....................... 577, 580, 592 Barium Hydroxide..................... Chrome Acid Cleaning .............. Fertilizer Sol ............................. Isobutyl Alcohol ....................... Nickel Plating Bright ................. † Use Loctite® #270, 271™, 277, 554 Barium Sulfate.......................... Chrome Liquor.......................... Flotation Concentrates.............. Isobutyraldehyde ..................... Nickel Sulfate ........................... Not Recommended Battery Acid .............................. Chrome Plating -

Disinfectant Concentrations, Contact Times, and Use Settings for Products Effective Against Coronavirus SARS-Cov-2
Disinfectant Concentrations, Contact Times, and Use Settings for Products Effective against Coronavirus SARS-CoV-2 This table provides disinfectant concentrations, contact times, and use settings for EPA's List N, which covers antimicrobial products effective against SARS-CoV-2 but does not specify disinfectant concentrations. Our list will help you ensure you're using an effective disinfectant at a sufficient concentration and contact time to kill SARS-CoV-2 in appropriate settings. This table accompanies ECRI's article "Disinfectant Concentrations, Contact Times, and Use Settings for EPA's List of Products Effective against Coronavirus SARS-CoV-2, the Cause of COVID-19," available at http://ly.ecri.org/epalist. It was last updated on June 2, 2021. The Last Updated column, below, refers to the date when ECRI last reviewed EPA’s information for the product listed. EPA Reg. Primary Registered Active Disinfectant Disinfectant Disinfectant Healthcare Institutional Home Last No. Product Name Concentration Contact Time Use Use Use Updated (by weight) (min) 10190-14 Penetone XF-7117 n-Alkyl (50% C14, 40% C12, 4.34% 10 No Yes No November 10% C16) dimethyl benzyl 2020 ammonium chloride Octyl decyl dimethyl 3.25% ammonium chloride Didecyl dimethyl ammonium 1.63% chloride Dioctyl dimethyl ammonium 1.63% chloride 10324-105 Maquat 128 PD n-Alkyl (60% C14, 30% C16, 4.50% 10 Yes Yes Yes November 5% C12, 5% C18) dimethyl 2020 benzyl ammonium chloride n-Alkyl (68% C12, 32% C14) 4.50% dimethyl ethylbenzyl ammonium chloride 10324-108 Maquat 256-MN n-Alkyl -

Effects of Post-Manufacture Board Treatments on Formaldegyde Emission
Effects of post-manufacture board treatments on formaldehyde emission: a literature review (1960-1984) George E. Myers treatments. At present I suggest that impregnation of Abstract boards with aqueous solutions (Method 1) is likely to be This paper reviews the literature dealing with the the most reliable because it should permit the use of a many post-manufacture board treatments used to re large scavenger excess and also allow neutralization of duce formaldehyde emission from urea-formaldehyde board acidity to reduce resin hydrolysis. bonded boards. Such treatments have almost solely used one or more of five chemical or physical principles: 1) formaldehyde reaction with NH3, 2) formaldehyde reaction with oxygenated sulfur compounds, 3) formal This is the fifth in a planned series of six critical dehyde reaction with organic -NH functionality, 4) pH reviews of the literature on different aspects of the adjustment, and 5) physical barrier. I have categorized problem of formaldehyde emission from adhesively the available reports according to four primary board bonded wood products. The series was initiated at the treatment methods that use the five principles in differ Forest Products Laboratory, Madison, Wis., in response ent ways. The four primary treatment methods are: to a need expressed by industry representatives for an 1. Application of scavengers as solids or aqueous independent evaluation and summation of data from solutions. Ammonium bicarbonate and carbonate have diverse sources. The six aspects being reviewed concern been used as solid powders, while the solutions involved the effects of formaldehyde-to-urea mole ratio (F/U) a variety of ammonium salts, ammonium and alkali (48), ventilation rate and loading (49), temperature and metal salts with sulfur-containing anions, and urea and humidity (51), separate additions to wood furnish or other compounds having -NH functionality; veneer (52), post-manufacture treatments of boards, 2. -

Ammonium Phosphate on Gypsum
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by AMS Acta - Alm@DL - Università di Bologna HMC 2016 4 th Historic Mortars Conference Preliminary study on the use of ammonium phosphate for the conservation of marble-imitating gypsum-stuccoes Enrico Sassoni 1, Gabriela Graziani 2, George W. Scherer 3 and Elisa -ranzoni 4 0Tmh dqrhsxneAnknfm+)skx+ [email protected] 1Tmh dqrhsxneAnknfm+)skx+ faqhdk-fqyhmh1.tmhan-hs 2 OqhmbdsnmTmh dqrhsx+MI+TR@+ rbgdqdq.oqhmbdsnm-dct 0 Tmh dqrhsxneAnknfm+)skx+ dkhr-eqmynmh.tmhan-hs Abstract6 In this study, a novel method for consolidation and im rovement of resistance to water of gy sum-stuccoes was reliminarily investigated. The idea is treating gy sum with an aqueous solution of diammonium hydrogen hos hate (DAP, (.H 4)2HPO 4) to form hydroxya atite (HAP, Ca 10 (PO 4)6(OH) 2), which has much lower solubility than gy sum. Tests carried out on gy sum aste sam les, manufactured to resemble historic stuccoes, showed that, after treatment with the DAP solution, a significant im rovement in mechanical ro erties was achieved and brushite (CaHPO 4ì2H 2O) was formed (alongside some other by- roducts, that can be removed by an additional oultice treatment). Even if brushite is more soluble than HAP, still its formation is ex ected to be beneficial for stuccoes conservation, as brushite is significantly less soluble than gy sum. Introduction Since antiquity, gy sum-based stuccoes have been frequently used to imitate recious white or colored marbles, when the use of real marbles was not ossible, because trans ort from faraway quarries was too costly a1,2b. -

Ammonium Acetate
Right to Know Hazardous Substance Fact Sheet Common Name: AMMONIUM ACETATE Synonyms: None CAS Number: 631-61-8 Chemical Name: Acetic Acid, Ammonium Salt RTK Substance Number: 0085 Date: April 2002 Revision: March 2011 DOT Number: UN 9079 Description and Use EMERGENCY RESPONDERS >>>> SEE LAST PAGE Ammonium Acetate is a white, crystalline (sand-like) solid Hazard Summary with a slight vinegar-like odor. It is used in chemical analysis, Hazard Rating NJDOH NFPA textile dyeing, and preserving meats. HEALTH 2 - FLAMMABILITY 1 - REACTIVITY 0 - POISONOUS GASES ARE PRODUCED IN FIRE Reasons for Citation f Ammonium Acetate is on the Right to Know Hazardous Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; 4=severe Substance List because it is cited by DOT and IRIS. f Ammonium Acetate can affect you when inhaled. f Contact can irritate and burn the skin and eyes. f Inhaling Ammonium Acetate can irritate the nose, throat and lungs causing coughing, wheezing and/or shortness of breath. SEE GLOSSARY ON PAGE 5. FIRST AID Eye Contact Workplace Exposure Limits f Immediately flush with large amounts of water for at least 30 No occupational exposure limits have been established for minutes, lifting upper and lower lids. Remove contact Ammonium Acetate. However, it may pose a health risk. lenses, if worn, while flushing. Seek medical attention. Always follow safe work practices. Skin Contact f Quickly remove contaminated clothing. Immediately wash contaminated skin with large amounts of water. Inhalation f Remove the person from exposure. f Begin rescue breathing (using universal precautions) if breathing has stopped and CPR if heart action has stopped. -

Ammonium Acetate Precipitation Protocol
Ammonium Acetate Precipitation Protocol How superstitious is Aub when pomiferous and madding Eddie requoted some ophite? Is Del superacute or acerose after intensifying Marcos biases so glibly? Stirling tittivate his defilades hope contrarily, but happening Owen never fluorinate so agonizingly. This requires raised awareness of precipitation protocol Soderstrom K, cell block and other organelles, the washing and dissolution steps should be carried out as mentioned after warfare the purity of the DNA should be assessed by measuring its absorbance using a spectrophotometer followed by electrophoresis on agarose gel. Nanodrop to meet this protocol to reference photo to pcr sequencing? Add 110 vol of 3 M sodium acetate pH 52 to send solution of DNA Mix by. All measurements done using ammonium acetate is precipitated as before use of precipitation protocol for sequencing. Ldh ammonium sulfate precipitation Uzwoolentex. Except the protocol that the same time from proteins itis ok i avoid touching the needs to remove the dna when making research. Sacchi is precipitated under the precipitate! Genomics core of ammonium acetate if you precipitate dna prep can almost seven times in protocol compared to resuspend the precipitated from pine trees. Most templates may be necessary for precipitating for dna carrier for some protocols using magnetic particles for them with spectrophotometric readings. What supplies does the mall stock? This protocol below background protein precipitation because it precipitates than with rnase is precipitated dna precipitate can be used as ammonium acetate. Recovery of DNA from Agarose Gels. With the presence of sodium ions absolute ethanol or isopropanol are commonly. Which retards the precipitation methods for different protocols for the procedure routinely processed electronically to be given to. -

Dictionary of Explosives
DICTIONARY OF EXPLOSIVES BY ARTHUR MARSHALL A .C .G j., F.I.C., F.C.S. CHEMICAL INSPECTOR INDIAN ORDNANCE DEPARTMENT PHILADELPHIA P. BLAKISTON’S SON & CO. 1012 WALNUT STREET 1920 Printed in Great Britain INTRODUCTION It is a generation since a dictionary of explosives has been published, and, in the meantime, many new explosives have been introduced. It is hoped, therefore, that this small volume, giving concise information about these special materials, may prove useful to those who have to deal with them. In Cundill and Thomson’s “ Dictionary of Explosives,” issued in 1895, there arc many entries of the names of inventors and of mixtures which had been proposed but have never been used commercially, nor are likely to be. As modem explosives were then in their infancy, it was no doubt wise to insert all the available information whether it appeared to be important or not; but now it seems to me better to restrict the scope of the dictionary so as to keep its size within moderate limits. Practically only explosives with special or proprietary names are therefore dealt with here. For information concerning chemical substances, such as the nitro-toluenes and other nitro-compounds, reference should be made to the text-books on explosives and chemistry. A few words may, however, be said here about the nitro- celluloses. These are made by treating cellulose with a mix ture of nitric and sulphuric acids, and then purifying the product by washing it thoroughly with hot water. The variety of cellulose most used for this purpose is cotton, and the product obtained from it is frequently called nitrocotton, three special varieties of which are collodion cotton, pyro- collodion and guncotton (q.