Final Program
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

OUTSTANDING WARRANTS As of 10/10/2017
OUTSTANDING WARRANTS as of 10/10/2017 AGUILAR, CESAR JESUS ALEXANDER, SARAH KATHEREN ALLEN, RYAN MICHAEL A AGUILAR, ROBERTO CARLOS ALEXANDER, SHARRONA LAFAYE ALLEN, TERRELL MARQUISE AARON, WOODSTON AGUILERA, ROBERTO ALEXANDER, STANLEY TOWAYNE ALLEN, VANESSA YVONNE ABABTAIN, ABDULLAH AGUILIAR, CANDIDO PEREZ ALEXANDER, STEPHEN PAUL ALMAHAMED, HUSSAIN HADI M MOHAMMED A AHMADI, PAULINA GRACE ALEXANDER, TERRELL ALMAHYAWI, HAMED ABDELTIF, ALY BEN AIKENS, JAMAL RAHEEM ALFONSO, MIGUEL RODRIGUEZ ALMASOUDI, MANSOUR ABODERIN, OLUBUSAYO ADESAJI AITKEN, ROBERT ALFORD, LARRY ANTONIO MOHAMMED ALMUTAIRI, ABDULHADI HAZZAA ABRAMS, TWANA AKIBAR, BRIANNA ALFREDS, BRIAN DANIEL ALNUMARI, HESHAM MOHSMMED ABSTON, CALEB JAMES AKINS, ROBERT LEE ALGHAMDI, FAHADAHMED-A ALONZO, RONY LOPEZ ACAMPORA, ADAM CHRISTOPHER AL NAME, TURKI AHMED M ALHARBI, MOHAMMED JAZAA ALOTAIBI, GHAZI MAJWIL ACOSTA, ESPIRIDION GARCIA AL-SAQAF, HUSSEIN M H MOHSEB ALHARBI, MOHAMMED JAZAA ALSAIF, NAIF ABDULAZIZ ACOSTA, JADE NICOLE ALASMARI, AHMAD A MISHAA ALIJABAR, ABDULLAH ALSHEHRI, MAZEN N DAFER ADAMS, ANTONIO QUENTERIUS ALBERDI, TOMMY ALLANTAR, OSCAR CVELLAR ALSHERI, DHAFER SALEM ADAMS, BRIAN KEITH ALBOOSHI, AHMED ABALLA ALLEN, ANDREW TAUONE ALSTON, COREY ROOSEVELT ADAMS, CHRISTOPHER GENE ALBRIGHT, EDMOND JERRELL ALLEN, ANTHONY TEREZ ALSTON, TORIANO ADARRYL ADAMS, CRYSTAL YVONNE ALCANTAR, ALVARO VILCHIS ALLEN, ARTHUR JAMES ALTMAN, MELIS CASSANDRA ADAMS, DANIEL KENNETH ALCANTAR, JOSE LUIS MORALES ALLEN, CHADWICK DONOVAN ALVARADO, CARLOS ADAMS, DARRELL OSTELLE ALCANTARA, JESUS ALLEN, CHRISTOPHER -

Baby Girl Names Registered in 2013
Page 1 of 48 Baby Girl Names Registered in 2013 # Baby Girl Names # Baby Girl Names # Baby Girl Names 1 Aadhya 1 Abbigayl 1 Acrom 1 Aadvika 1 Abbrianna 18 Ada 1 Aadya 17 Abby 1 Adabell 1 Aaesha 3 Abbygail 1 Adahlia 1 Aafarida 2 Abbygale 1 Adair 2 Aahana 1 Abeeha 1 Adalaya 1 Aahna 1 Abeera 1 Adalayne 1 Aaiana 2 Abegail 2 Adalee 2 Aaira 1 Abelia 1 Adaleyah 1 Aaisha 1 Abemael 1 Adali 1 Aalaiyah 1 Aberdeen 4 Adalia 1 Aaleen 1 Abha 1 Adalina 2 Aaleyah 1 Abhitha 1 Adaline 5 Aaliya 2 Abi 17 Adalyn 42 Aaliyah 1 Abiah 19 Adalynn 1 Aaliyah-Faye 3 Abigael 4 Adalynne 1 Aamina 164 Abigail 2 Adama 3 Aaminah 1 Abigail-Crystal 1 Adamay 2 Aamna 2 Abigale 1 Adan 9 Aanya 1 Abigayl 3 Adanna 1 Aara 4 Abigayle 3 Adara 3 Aaradhya 1 Abigia 1 Adarah 1 Aaralee 1 Abiha 1 Adasyn 1 Aaralynn 1 Abira 1 Adaya 1 Aaria 1 Abisatou 2 Addalyn 1 Aarieanna 1 Abisha 1 Addalynn 1 Aarika 3 Abrianna 1 Addasyn 4 Aarna 1 Abrie 1 Addelinn 1 Aarohi 1 Abriela 1 Addelyn 1 Aarushi 2 Abriella 3 Addie 5 Aarya 7 Abrielle 1 AddieMae 1 Aaseas 1 Abril 1 Addilia 1 Aashna 1 Abryanna 1 Addiline 1 Aasia 1 Abul 2 Addilyn 1 Aasiya 1 Aby 1 Addin 1 Aavry 1 Abyelle 1 Addington 1 Aayah 2 Abygail 1 Addisen 1 Aayat 1 Abygeil 74 Addison 1 Aayla 3 Acacia 7 Addisyn 1 Aazeen 3 Acadia 3 Addley 1 Abagael 1 Acelin 11 Addyson 2 Abagail 1 Acelynn 1 Adeeb 1 Abaigeal 1 Achad 3 Adeena 4 Abbey 1 Achan 1 Adeife 1 Abbi 1 Achette 2 Adela 3 Abbie 1 Achiraya 1 Adelaida 1 Abbiegale 1 Achoi 13 Adelaide 7 Abbigail 1 Achok 8 Adele 1 Abbigaile 1 Achols 1 Adelène 1 Abbigale 1 Acil 1 Adelie Page 2 of 48 Baby Girl Names Registered -

Tonya Pinkins
tonya pinkins October 2008 most actors spend a lifetime dreaming of career success. According to Tonya Pinkins, success was her undoing. In 1992 Pinkins’s career was taking off . She was appearing on the soap opera, As the World Turns , and she had just won rave reviews and a Tony Award for Jelly’s Last Jam . That success, says Pinkins, was too much for the ego of her then-husband. “When you’re coming from a house full of women,” she says, “you really don’t know anything about men and have no basis for picking and choosing. I just picked a string of really bad ones.” This one not only sued for divorce; he fought for and won sole custody of Pinkins’s two children. She had to leave the show to focus on the court proceedings, spent years in battle, going bank- rupt in the process, and found that for the rest of her career to date, despite artistic success, she’d be dealing with the fallout. Tonya Pinkins was born in Chicago into a home inhabited by her mother, grandmother, great-grandmother and aunts, none of whom were especially interested in the arts. A junior high school teacher saw Pinkins’s talent, however, and lobbied the St. Nicholas Theater Com- pany to allow her to study in their professional program. By nineteen she was in New York, having been cast in the legendary fl op, Merrily We Roll Along . Her next Broadway musical was Jelly’s Last Jam , followed by the disappointments, Chronicles of a Death Foretold, Play On! , and The Wild Party . -

Architecture As Experience
Architectural Research in Finland, vol.2, no.1 (2018) 9 Architecture as Experience The fusion of the world and the self Juhani Pallasmaa Aalto University (professor emeritus) [email protected] Abstract The complex phenomenon of architecture consists of too many irreconcilable and conflicting categories of thought, intention, emotion, interaction and action to be condensed into the framework of a single theory of architecture. Besides, art and architecture are constituted in their mental encounter and experience instead of the material works themselves. Works of architecture and art are encountered and lived rather than understood intellectually. Architecture is commonly understood, taught, practiced and evaluated primarily as a visual art form. However, we encounter buildings and environments through our entire sense of being. Perceptions interact with memory and imagination to constitute an experience with meaning and temporal duration. Art and architecture are essentially relational phenomena as they express our being in the world instead of themselves or their authors. The interest in architecture as experience also directs our attention to such diffuse and neglected experiences as atmospheres, ambiences, feelings, moods and attunements. Keywords: experience, existential sense, relational phenomena. Introduction Modern architectural theory, education and practice have regarded architecture as visually aestheticised spaces and material structures, and primarily studied their historical, functional, technical and formal characteristics. The analyses have focused on architecture as physical objects and spaces and their geometric and compositional qualities, as well as the representation of these properties in drawings. As architecture does not possess a comprehensive theory of its own, the point of view and method of research have usually been borrowed from other disciplines in accordance with changing interests and fashions; often the applicability of the chosen theoretical frameworks have been highly questionable in the specific reality of architecture. -

Class of 2020
Class of 2020 Byblos Campus Academic Officers President Dr. Joseph G. Jabbra Provost Dr. Georges E. Nasr School of Arts & Sciences Dr. Cathia Jenainati Dean Adnan Kassar School of Business Dr. Wassim Shahin Dean School of Architecture & Design Dr. Elie Haddad Dean School of Engineering Dr. Lina Karam Dean School of Pharmacy Dr. Imad Btaiche Dean Alice Ramez Chagoury Dr. Costantine Daher School of Nursing Interim Dean Gilbert and Rose-Marie Chagoury Dr. Michel E. Mawad School of Medicine Dean ALMA MATER We greet thee, O College fair, In the Shadow of Lebanon, Where its mystic haze doth melt In the Mediterranean sea. CHORUS: Alma Mater, hail to thee, Receive our pledge of constancy Alma Mater, hail to thee, Deep loyalty we vow. To thy portals, O College fair, We thy students have come from far, We have answered thy call to dare, To adventure beneath thy star. By the light of thy holy fires, We shall follow the way of truth; In the strength of our new desires, We pledge to thee all our youth. (Sung to the tune of “Follow the Gleam”) Awardees 2019- 2020 School of Arts & Sciences Jana Oussama Dib El Jalbout Valedictorian Christelle Elias Barakat President Award Rhea Ghazy Wakim Torch Award and Rhoda Orme Award Adnan Kassar School of Business Wendy Toni El Hage President Award Jude Manhal Darwich Torch Award School of Architecture & Design Rihab Maan Soukkarieh President Award Georges Antoine Eid Torch Award School of Engineering Joelle Bachir El Sayegh President Award Johnny Joseph Sawma Awad Torch Award Joseph Elias Chakar The Charbel Khairallah -

Antonius Andreae Scotism's Best Supporting Auctor
ANTONIUS ANDREAL SCOTISM'S BEST SUPPORTING AUCTOR Marek GF.NSLLR I. THE MAKING OF A DOCTOR DULCIFLUUS ANTONIUS ANDREAE AND HIS POSITION IN FORMATION OF SCOTISM The turning of the 13th and 14th century saw the emergence of a bright new star on the firmament of philosophy - the man who reinvented scho- lasticism after the Condemnation of 1277: John Duns Scotus. The light of his thought soon lit up inspiration in many adepts of the Liberal Arts and the gravity of his doctrine had caught, for some time at least, a number of young thinkers, who later became most influential philosophers of 14th century, like Francis de Mayronis, William of Ockham and Peter Auriol, to name a few. For most of them, however, the contact with the doctrine of the Subtle Doctor was only an inspiring episode early in their careers and it would be difficult to call them advocates of scotism, much less ,,true,, scotists. And still, despite the fact that so many of Scotus' best pu- pils later turned their backs on his teaching, despite Scotus' early death that had left his work unfinished, the doctrine of John Duns not only sur- vived but started flourishing and gradually became one of the most vital and powerful philosophical schools of later middle ages, whose influence was still felt at the universities even in the 18th century. How was it possi- ble that the ideas so subtle and so sketchy won minds and hearts of so many lovers of wisdom? Propagation of Scotus' views required devout followers, skilful inter- preters and commentators and, last but not least, good thinkers who, ac- cepting the opinions of John Duns for their own, would develop them in- to a comprehensive doctrine covering the whole spectrum of philosophical inquiry of the time, in a word: transform them into scotism. -

Cemetery-Burials by Last Name
Cemetery - Burials By Last Name Last Name First Name Middle Maiden Name Section Block Lot Grave Died Age Burial Date AARONS PATRICIA LYNN B 214 D 8/20/2010 AASEN CARL MASONIC 119 2 2 3/2/1983 83 AASEN FLORENCE LUCILLE MASONIC 119 4 2/21/1990 66 2/24/1990 AASEN JUDITH MASONIC 119 3 3 3/7/1977 78 ABBE IVAN ROBERT J 2 8 A 7/2/1953 24 ABBOTT CHARLES A 78A 5 6/4/1912 28 ABBOTT GARY DELBERT NATURES PATHWAY E 19 1/7/2015 70 1/23/2015 ABBOTT KETURAH OLD CEMETERY 368 4/23/1907 0 ABEL MAGDALINE OLD CEMETERY 97 2/13/1905 0 ABERNATHY CLYDE C. A 74A 5 1/16/1957 77 ABERNATHY EDGAR W. I 6 8 D 8/14/1976 68 ABERNATHY IMA I 6 8 E 7/25/1980 72 ACHILLES ALBERT O. D 14 8 A 12/21/1963 0 ACHILLES FRED A 93 4 9/2/1920 71 ACHILLES MARY D 14 8 B 5/22/1965 74 ACHILLES OLGA A 93 5 7/5/1937 84 ACHORN OLIVER OLD CEMETERY 397 2/28/1894 0 ACKER JOHN A 7 4 8/12/1917 34 ACKER JOHN OLD CEMETERY 72 1/26/1901 0 ACKERMAN BERNICE LOIS J 2 8 B 11/12/1988 86 11/16/1988 ADAMS BRANDON J. J 12 1 N 9/14/1974 0 Friday, March 23, 2018 Page 1 of 690 Last Name First Name Middle Maiden Name Section Block Lot Grave Died Age Burial Date ADAMS ELIAS E 1 4 A 11/27/1923 73 ADAMS HARRIET B 227 C 10/22/1958 81 ADAMS INA CHASE B 100 D 8/22/1950 72 ADAMS LLEWELLYN B 100 C 9/14/1936 63 ADAMS MIRIAM REEL L 3 15 A 11/4/2014 95 11/13/2014 ADAMS NELLIE OR MILLIE 1ST ADDITION 182 5 9/13/1913 0 ADAMS PAMELA J. -
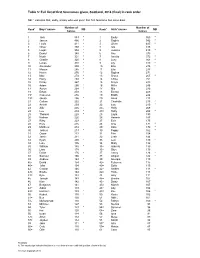
Table 5: Full List of First Forenames Given, Scotland, 2014 (Final) in Rank Order
Table 5: Full list of first forenames given, Scotland, 2014 (final) in rank order NB: * indicates that, sadly, a baby who was given that first forename has since died. Number of Number of Rank1 Boys' names NB Rank1 Girls' names NB babies babies 1 Jack 583 * 1 Emily 569 * 2 James 466 * 2 Sophie 542 * 3 Lewis 411 * 3 Olivia 485 * 4 Oliver 403 * 4 Isla 435 5 Logan 354 * 5 Jessica 419 * 6 Daniel 349 * 6 Ava 379 7 Noah 321 * 7 Amelia 372 * 8 Charlie 320 * 8 Lucy 363 * 9 Lucas 310 * 9 Lily 310 * 10 Alexander 309 * 10 Ellie 278 * 11 Mason 285 * 11 Ella 273 12 Harris 276 * 12 Sophia 271 13 Max 274 * 13 Grace 265 * 14 Harry 268 * 14 Chloe 251 15 Finlay 267 15 Freya 249 16 Adam 266 * 16 Millie 246 17 Aaron 264 * 17 Mia 230 18 Ethan 259 18 Emma 229 19= Cameron 256 19 Eilidh 224 19= Jacob 256 * 20 Anna 218 21 Callum 252 21 Charlotte 213 * 22 Archie 239 22 Eva 210 * 23 Alfie 236 * 23= Holly 208 24 Leo 234 * 23= Ruby 208 * 25 Thomas 228 * 25 Layla 190 26 Nathan 226 26 Hannah 187 27 Riley 223 * 27 Evie 175 28 Rory 215 28 Orla 171 * 29 Matthew 214 29 Katie 170 * 30 Joshua 213 * 30 Poppy 162 * 31 Oscar 212 31 Erin 154 32 Jamie 211 32 Leah 144 33 Ryan 208 * 33 Lexi 140 * 34 Luke 195 34 Molly 132 35 William 180 * 35= Isabella 130 36 Liam 178 35= Skye 130 37 Dylan 176 * 37 Lacey 124 * 38 Samuel 166 38 Abigail 122 * 39 Andrew 163 * 39 Georgia 119 40= David 154 * 40= Rebecca 115 40= John 154 40= Sofia 115 42 Connor 146 42= Amber 113 * 43= Brodie 144 42= Hollie 113 43= Kyle 144 * 44 Amy 111 45 Joseph 143 45= Brooke 107 46 Kian 142 * 45= Daisy 107 47 Benjamin 141 45= Niamh 107 48= Aiden 139 48= Lilly 106 48= Harrison 139 * 48= Zoe 106 * 50 Robert 135 * 50 Rosie 102 51= Ben 130 51 Abbie 101 51= Muhammad 130 52 Robyn 100 * 53 Michael 127 53 Sienna 98 54 Tyler 123 * 54= Summer 96 55 Kai 120 54= Zara 96 56 Euan 114 * 56 Iona 91 57= Arran 112 57 Maya 89 57= Jayden 112 58 Sarah 88 59 Jake 111 * 59= Aria 87 60= Cole 110 59= Maisie 87 60= Ollie 110 * 61 Cara 85 Footnote 1) The equals sign indicates that there is more than one name with this position of rank in the list. -

2011 Annual Report
2011 ANNUAL REPORT 1 CARING. PRACTICE. COLLABORATION. COMMUNICATION. PROFESSIONAL DEVELOPMENT. 2. Leadership Message 5. Highlights—At a Glance 17. WE PROVE Every Day That “Impossible” Is Just an Opinion 18. WE GO Beyond Traditional Thinking 19. WE PUSH Medicine Further than Ever Before 20. WE APPLY Science and Creativity to Our Thinking 22. WE TURN Ideas into Reality 24. Collaboration in the Pursuit of Excellence 26. We Are Committed to Providing High-Quality Clinical Care IBC. In Fond Memory of Debra Lione, RN HacKensacK University MEDical Center’S Vision To Lead the Pursuit of Excellence in Healthcare Nursing’S Vision Pursuing Excellence in Professional Nursing Practice and Care Delivery HacKensacK University MEDical Center’S Statement OF Purpose Hackensack University Medical Center is a team committed to providing an exceptional patient experience through quality patient-centered care, education, research and community outreach. Hackensack University Medical Center, a nonprofit teaching and research hospital located in Bergen County, New Jersey, is the largest provider of inpatient and outpatient services in the state. This 775-bed facility has gone beyond traditional thinking by creating an entire campus of care, including: the Heart & Vascular Hospital, the John Theurer Cancer Center, the Joseph M. Sanzari Children’s Hospital, and the Donna A. Sanzari Women’s Hospital. As a result of using science and creativity to push medicine further, HackensackUMC has been named one of America’s 50 Best Hospitals by HealthGrades® for six years in a row—the only hospital in New Jersey, New York, and New England to receive this honor. The medical center has been named to the Leapfrog Top Hospitals List; received 17 Gold Seals of Approval™ by the Joint Commission; is ranked in Cancer, Cardiology and Heart Surgery, Ear, Nose & Throat, Gastroenterology, Geriatrics, Neurology and Neurosurgery, Orthopedics and Urology by U.S. -

Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty Springer-Verlag France S.A.R.L Jean-Alain Epinette, MD Michael T
Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty Springer-Verlag France S.A.R.L Jean-Alain Epinette, MD Michael T. Manley, PhD Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty Preface by Rudolph G.T. Geesink, MD, PhD Springer Jean-Alain Epinette, MD Clinique Medico-Chirurgicale 200, rue d'Auvergne 62700 Bruay-Labuissiere France Michael T. Manley, PhD 12-A Chestnut Street Ridgewood, NJ 07450 USA ISBN 978-2-287-00508-4 ISBN 978-2-8178-0851-2 (eBook) DOI 10.1007/978-2-8178-0851-2 © Springer-Verlag France 2004 Originally published by Springer Paris Berlin Heidelberg New York in 2004 Apart from any fair dealing for the purposes of the research or private study, or criticism or review, as permitted under the Copyright, Designs and Patents Act 1998, this publication may only be reproduced, stored or transmitted, in any form or by any means, with the prior permission in writing of the publishers, or in the case of reprographic reproduction in accordance with the terms of licenses issued by the copyright. Enquiry concerning reproduction outside those terms should be sent to the publishers. The use of registered names, trademarks, etc, in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant laws and regulations and therefore free for general use. Product liability: the publisher can give no guarantee for information about drug dosage and application thereof contained in this book. In evety individual case, the user must check its accuracy by consulting other pharmaceuticalliterature. -

2017 Purge List LAST NAME FIRST NAME MIDDLE NAME SUFFIX
2017 Purge List LAST NAME FIRST NAME MIDDLE NAME SUFFIX AARON LINDA R AARON-BRASS LORENE K AARSETH CHEYENNE M ABALOS KEN JOHN ABBOTT JOELLE N ABBOTT JUNE P ABEITA RONALD L ABERCROMBIA LORETTA G ABERLE AMANDA KAY ABERNETHY MICHAEL ROBERT ABEYTA APRIL L ABEYTA ISAAC J ABEYTA JONATHAN D ABEYTA LITA M ABLEMAN MYRA K ABOULNASR ABDELRAHMAN MH ABRAHAM YOSEF WESLEY ABRIL MARIA S ABUSAED AMBER L ACEVEDO MARIA D ACEVEDO NICOLE YNES ACEVEDO-RODRIGUEZ RAMON ACEVES GUILLERMO M ACEVES LUIS CARLOS ACEVES MONICA ACHEN JAY B ACHILLES CYNTHIA ANN ACKER CAMILLE ACKER PATRICIA A ACOSTA ALFREDO ACOSTA AMANDA D ACOSTA CLAUDIA I ACOSTA CONCEPCION 2/23/2017 1 of 271 2017 Purge List ACOSTA CYNTHIA E ACOSTA GREG AARON ACOSTA JOSE J ACOSTA LINDA C ACOSTA MARIA D ACOSTA PRISCILLA ROSAS ACOSTA RAMON ACOSTA REBECCA ACOSTA STEPHANIE GUADALUPE ACOSTA VALERIE VALDEZ ACOSTA WHITNEY RENAE ACQUAH-FRANKLIN SHAWKEY E ACUNA ANTONIO ADAME ENRIQUE ADAME MARTHA I ADAMS ANTHONY J ADAMS BENJAMIN H ADAMS BENJAMIN S ADAMS BRADLEY W ADAMS BRIAN T ADAMS DEMETRICE NICOLE ADAMS DONNA R ADAMS JOHN O ADAMS LEE H ADAMS PONTUS JOEL ADAMS STEPHANIE JO ADAMS VALORI ELIZABETH ADAMSKI DONALD J ADDARI SANDRA ADEE LAUREN SUN ADKINS NICHOLA ANTIONETTE ADKINS OSCAR ALBERTO ADOLPHO BERENICE ADOLPHO QUINLINN K 2/23/2017 2 of 271 2017 Purge List AGBULOS ERIC PINILI AGBULOS TITUS PINILI AGNEW HENRY E AGUAYO RITA AGUILAR CRYSTAL ASHLEY AGUILAR DAVID AGUILAR AGUILAR MARIA LAURA AGUILAR MICHAEL R AGUILAR RAELENE D AGUILAR ROSANNE DENE AGUILAR RUBEN F AGUILERA ALEJANDRA D AGUILERA FAUSTINO H AGUILERA GABRIEL -

Babies' First Forenames: Births Registered in Scotland in 1993
Babies' first forenames: births registered in Scotland in 1993 Information about the basis of the list can be found via the 'Babies' First Names' page on the National Records of Scotland website. Boys Girls Position Name Number of babies Position Name Number of babies 1 Andrew 1099 1 Emma 794 2 Ryan 922 2 Rebecca 755 3 David 908 3 Lauren 703 4 Scott 848 4 Laura 677 5 James 791 5 Amy 643 6 Michael 784 6 Sarah 603 7 Christopher 780 7 Nicole 585 8 Ross 766 8 Rachel 555 9 Craig 706 9 Stephanie 481 10 Jordan 664 10 Hannah 464 11 Daniel 641 11 Jennifer 463 12 Jamie 622 12 Kirsty 449 13 John 554 13 Megan 443 14 Steven 513 14 Danielle 409 15 Sean 488 15 Claire 387 16 Mark 478 16 Nicola 376 17 Liam 446 17 Samantha 369 18 Lewis 407 18 Gemma 362 19 Stuart 406 19 Lisa 333 20= Matthew 396 20 Natalie 321 20= Paul 396 21 Louise 298 22 Thomas 394 22 Jade 280 23= Robert 391 23 Ashley 270 23= Stephen 391 24 Shannon 267 25 Connor 387 25 Chloe 259 26 Callum 385 26= Caitlin 230 27 Cameron 356 26= Fiona 230 28 Darren 343 28 Hayley 221 29 Calum 334 29 Kayleigh 219 30 Jack 321 30= Katie 218 31 Gary 312 30= Rachael 218 32 Adam 303 32 Melissa 217 33 Alexander 302 33 Sophie 213 34 William 299 34 Heather 212 35 Kieran 292 35 Ashleigh 210 36 Kyle 276 36 Emily 190 37 Jonathan 260 37 Natasha 188 38 Fraser 250 38 Amanda 185 39 Grant 249 39 Chelsea 184 40 Dean 240 40= Eilidh 182 41 Kevin 237 40= Lucy 182 42 Shaun 235 42 Stacey 180 43 Martin 229 43 Kelly 172 44 Lee 220 44 Jodie 168 45 Alan 174 45 Robyn 163 46 Iain 171 46= Kerry 159 47 Gavin 162 46= Victoria 159 48 Peter