The Chalk Aquifer of Hampshire
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

River Test – Tufton
River Test – Tufton An advisory visit carried out by the Wild Trout Trust – April 2009 1. Introduction This report is the output of a Wild Trout Trust advisory visit undertaken on the River Test at Tufton near Whitchurch in Hampshire. The advisory visit was carried out at the request of the Hampshire Wildlife Trust. The Trust is looking at various options for enhancing local biodiversity and exploring possible habitat enhancement opportunities under Higher Level Stewardship agreements with the landowners. Throughout the report, normal convention is followed with respect to bank identification i.e. banks are designated Left Bank (LB) or Right Bank (RB) whilst looking downstream. 2. Catchment overview The River Test is nationally recognised as the quintessential chalk river and is designated for most of its length as a Site of Special Scientific Interest (SSSI). The Test has a world-wide reputation for being a first class trout (Salmo trutta) fishery. Much of the middle and lower river is heavily stocked with hatchery derived trout to support intense angling activity. Where good quality habitats are maintained the river has the capacity to produce viable numbers of wild fish. A major bottleneck to enhanced wild production is thought to be through poor in- gravel egg survival. Comparatively small areas of nursery habitat also restrict the development of wild stocks. Where decent habitats are found and preserved, survival rates of fry are usually superb due to rapid growth rates. Habitat quality on the Test varies enormously. The river channels are virtually all heavily modified, artificial and originally constructed for power generation or water meadow irrigation. -

The Ramblers' Association Working for Walkers
Portsmouth Group Summer - Autumn (July – October 2018) Walks Programme The Ramblers' Association Working for Walkers The RA promotes rambling, protects Rights of Way, campaigns for access to open country and defends the beauty of the countryside Web site: Head office www.ramblers.org.uk Local http://www.portsmouthramblers.org.uk/ Email: Head office [email protected] Local Local [email protected] PORTSMOUTH GROUP WALKING PROGRAMME Correct as at 30th MAY 2018. Check the website for any updates. The Portsmouth Group offers a variety of walks mainly in Hampshire, West Sussex and the Isle of Wight. On Saturdays these are moderately paced 5 to 9 mile walks whilst, on Sundays, they are faster paced 11 to 15 mile walks. The Group also has short Friday evening walks and some extra Saturday walks in more distant parts in the summer months. Most walks have a pub/cafe stop either at the end or at lunch time. New walkers always welcome. Just turn up at meeting place for shorter walks. For walks in excess of 10 miles, please contact leader first if you have not previously walked this distance. Grade A walks – for experienced walkers; brisk pace Grade B walks – regular pace Grade C walks – moderate pace Grade D walks – slow pace These are only general indications of the pace and will vary with the individual leader. Portsmouth Group Summer - Autumn (July - October 2018) Programme Page 2 Never rely on this printed programme. Check the Portsmouth Ramblers website for any changes. CAR SHARING AND LIFTS– IMPORTANT INFORMATION Our group encourages car sharing as well as assisting those without transport and those with transport but who lack confidence driving to unfamiliar areas to get to the walks. -

South East River Basin District Flood Risk Management Plan 2015 - 2021 PART B: Sub Areas in the South East River Basin District
South East River Basin District Flood Risk Management Plan 2015 - 2021 PART B: Sub Areas in the South East river basin district March 2016 Published by: Environment Agency Further copies of this report are available Horizon house, Deanery Road, from our publications catalogue: Bristol BS1 5AH www.gov.uk/government/publications Email: [email protected] or our National Customer Contact Centre: www.gov.uk/environment-agency T: 03708 506506 Email: [email protected]. © Environment Agency 2016 All rights reserved. This document may be reproduced with prior permission of the Environment Agency. Contents Glossary and abbreviations ......................................................................................................... 5 The layout of this document ........................................................................................................ 7 1 Sub-areas in the South East river basin district .............................................................. 9 Introduction ................................................................................................................................. 9 Flood Risk Areas ......................................................................................................................... 9 Management catchments ............................................................................................................ 9 2 Conclusions, objectives and measures to manage risk for the Brighton and Hove Flood Risk Area.......................................................................................................................... -

Act 1990 LIST of NEW PLANNING and OTHER
Town and Country Planning Acts 1990 Planning (Listed Building and Conservation Area) Act 1990 LIST OF NEW PLANNING AND OTHER APPLICATIONS, RECEIVED AND VALID IN PARISH/TOWN WARD ORDER WEEKLY LIST AS AT 06 May 2012 For Guidance 1 Those applications which have a star by them are FOR INFORMATION PURPOSES ONLY. Please see over for a list and description of application types. 2 If the Grid Reference is blank, this is because it is a property which is in the process of being entered on the Property Database for the District. 3 The Conservation Area only appears where the application is known to be in a Conservation Area . 4 Where the application has not been allocated to a case officer, “Team Manager “ for the relevant area appears instead. 5 The Publicity Period is 21 days after the Site Notice Date or 21 days after a Press Advert date, where this has been made, whichever is the later. Applications may be determined after this date. If you require any further information, please enquire at Main Reception, City Offices, Colebrook Street, Winchester, SO23 9LJ quoting the Case Number. We will then be able to give you the current status of the application. We are open Monday to Thursday 8.30am - 5.00pm, Fridays 8.30am - 4.30pm, excluding Bank and Public Holidays. Page 1 APPLICATION CODES AND DESCRIPTION AVC Full Advert Consent AVT Temporary Advert Consent (non-standard time limit) AGA Agricultural Application APN Agricultural Prior Notification CER Certificate of Alternative Use CHU Change of Use DEC Demolition Consultation EUC Established Use -

Draft Water Resources Management Plan 2019 Annex 14: SEA Main Report
Draft Water Resources Management Plan 2019 Annex 14: SEA Main Report Appendix A: Consultee responses to the scoping report and amendments made as a consequence November 30, 2017 Version 1 Appendix A Statement of Response Southern Water issued its Strategic Environmental Assessment (SEA) Scoping Report for its Draft Water Resources Management Plan 2019 for public consultation from 28th April 2017 to 2nd June 2017. Comments on the SEA Scoping Report were received from the following organisations: Natural England Environment Agency Historic England Howard Taylor, Upstream Dry Fly Sussex Wildlife Trust The Test & Itchen Association Ltd Wessex Chalk Stream Rivers Trust Forestry Commission England Hampshire and Isle of Wight Wildlife Trust Longdown Management Limited Amanda Barker-Mill C. H. Layman These comments are set out in Table 1 together with Southern Water’s response as to how it intends to take account of them in developing the SEA of the Draft Water Resources Management Plan. Table 1 Draft Water Resources Management Plan: SEA Scoping Report – responses to comments received How comments have been addressed in the Ref Consultee Comment Draft Water Resources Management Plan Environmental Report Plans programmes or policies I recommend you add the following to your list of plans programmes or policies: National. - Defra strategy for the environment creating a great place for These policies, plans and programmes have Natural living. been included in the SEA Environmental Report 1 England - The national conservation strategy conservation-21 and considered in the assessment of potential effects of the WRMP. - The 5 point plan to salmon conservation in the UK National Nature Reserve Management Plans (though you may not be able to, or need to, list all of these, please just reference them as a source of information for assessment of any relevant options). -

Hamble River Logboat: Report on Recent Investigation by HWTMA
Hamble River Logboat: Report on Recent Investigation by HWTMA Hampshire and Wight Trust for Maritime Archaeology September 2010 Hamble River Logboat Study Report Contents I. DOCUMENT CONTROL ........................................................................................................... 1 II. LIST OF FIGURES & TABLES .................................................................................................. 1 III. ACKNOWLEDGEMENTS......................................................................................................... 2 1. BACKGROUND .................................................................................................................... 2 1.1 PROJECT AIMS AND OBJECTIVES ........................................................................................ 2 1.2 THE RIVER HAMBLE ........................................................................................................... 2 1.3 HISTORY OF THE HAMBLE LOGBOAT ................................................................................... 2 1.4 THE HAMBLE LOGBOAT TODAY............................................................................................ 5 2. INVESTIGATION OF THE HAMBLE LOGBOAT................................................................. 6 2.1 DENDRO-CHRONOLOGY (BY NIGEL NAYLING)...................................................................... 6 3. ANALYSIS OF THE HAMBLE LOGBOAT........................................................................... 7 3.1 CONTEXT ......................................................................................................................... -

A Model for the Evolution of the Weald Basin
A model for the evolution of the Weald Basin DAVID L. HANSEN, DEREK J. BLUNDELL & S0REN B. NIELSEN Hansen, D.L., Blundell, D.J. & Nielsen, S.B. 2002-12-02. A Model for the evolution of the Weald Basin. Bulletin of the Geological Society of Denmark, Vol. 49, pp. 109-118. Copenhagen. https://doi.org/10.37570/bgsd-2003-49-09 The Weald Basin developed through the Jurassic-Lower Cretaceous as an extensional basin founded upon E-W trending low-angle faults that were probably Variscan thrusts, subsequently reactivated as normal faults. Later, the basin was inverted and uplifted into a broad dome, whilst the London Basin to the north, and the Hampshire-Dieppe Basin to the south, subsided as flanking basins during the late Palaeocene-Eocene. Seismic sections across the Weald indicate that inversion resulted from north-directed stress. A stratigraphic reconstruction based on a N-S profile across the Weald and flanking basins serves as a template for a forward, 2D thermo-mechanical model that simulates the evolution of the Weald Basin through crustal extension and its inversion, and subsidence of the flanking basins, through compression. The model provides a physical explanation for this sequence of events, requiring a region of crust of reduced strength relative to its flanks. This weak region is the location of crustal-scale Variscan thrusts that have been reactivated subsequently. The strong crust on the flanks is essential for the development of flanking basins during inversion and uplift of the Weald. Keywords: Basin inversion, lithosphere, thermo-mechanical modelling, finite elements, visco-elas tic-plastic, sedimentation, erosion. -
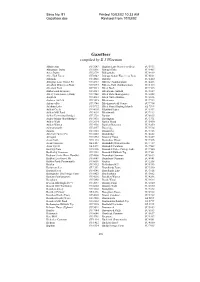
Gazetteer.Doc Revised from 10/03/02
Save No. 91 Printed 10/03/02 10:33 AM Gazetteer.doc Revised From 10/03/02 Gazetteer compiled by E J Wiseman Abbots Ann SU 3243 Bighton Lane Watercress Beds SU 5933 Abbotstone Down SU 5836 Bishop's Dyke SU 3405 Acres Down SU 2709 Bishopstoke SU 4619 Alice Holt Forest SU 8042 Bishops Sutton Watercress Beds SU 6031 Allbrook SU 4521 Bisterne SU 1400 Allington Lane Gravel Pit SU 4717 Bitterne (Southampton) SU 4413 Alresford Watercress Beds SU 5833 Bitterne Park (Southampton) SU 4414 Alresford Pond SU 5933 Black Bush SU 2515 Amberwood Inclosure SU 2013 Blackbushe Airfield SU 8059 Amery Farm Estate (Alton) SU 7240 Black Dam (Basingstoke) SU 6552 Ampfield SU 4023 Black Gutter Bottom SU 2016 Andover Airfield SU 3245 Blackmoor SU 7733 Anton valley SU 3740 Blackmoor Golf Course SU 7734 Arlebury Lake SU 5732 Black Point (Hayling Island) SZ 7599 Ashlett Creek SU 4603 Blashford Lakes SU 1507 Ashlett Mill Pond SU 4603 Blendworth SU 7113 Ashley Farm (Stockbridge) SU 3730 Bordon SU 8035 Ashley Manor (Stockbridge) SU 3830 Bossington SU 3331 Ashley Walk SU 2014 Botley Wood SU 5410 Ashley Warren SU 4956 Bourley Reservoir SU 8250 Ashmansworth SU 4157 Boveridge SU 0714 Ashurst SU 3310 Braishfield SU 3725 Ash Vale Gravel Pit SU 8853 Brambridge SU 4622 Avington SU 5332 Bramley Camp SU 6559 Avon Castle SU 1303 Bramshaw Wood SU 2516 Avon Causeway SZ 1497 Bramshill (Warren Heath) SU 7759 Avon Tyrrell SZ 1499 Bramshill Common SU 7562 Backley Plain SU 2106 Bramshill Police College Lake SU 7560 Baddesley Common SU 3921 Bramshill Rubbish Tip SU 7561 Badnam Creek (River -

Briefing Note for Ward Councillors and Chair of South Wonston Parish Council
Briefing Note for Ward Councillors and Chair of South Wonston Parish Council Affordability Housing in South Wonston both to buy and privately rent is expensive when compared to average incomes. Average house prices in South Wonston are in the region of 12 times average incomes. (Source: ONS / WCC) Many people will be unable to ever buy a property, or rent without assistance. The insinuation that all should see eventually buying a home in the village as a possibility shows a mis-understanding of the scale of the affordability problem. Right Move on 23 October 2018 stated from Land Registry Sold Homes information that the average price to buy a home in South Wonston was in the region of £412,429 (ONS 2018 Average District price £365,000). Across Winchester District: o Individual Lower Quartile/Average incomes £26k/£36k (Source ONS 2018) o Lower Quartile Household £39k/ £46k (ONS/OECD Modelled for 2 adults/2 adults + child 2018) o With Lower Quartile prices across District @ £290k (ONS 2018) homes are unaffordable o In the last 10 years across the District house prices are up 40%; incomes 18%; affordability ratio worsened from over 9% to over 11% (England 7.25%). Improving Affordability City Council objective to see more affordable housing provided both in urban and rural areas. Embraced by many parishes. E.g. Scheme close to completion in Wonston. Completed scheme in Micheldever. Both are interested in additional provision 40 rural schemes in Winchester and 50 villages assisted across HARAH (2000 people housed) 99% of new homes go to local people Important to provide homes in rural areas – A rural exception site is very different from a site like Kings Barton, in Winchester. -

Streams, Ditches and Wetlands in the Chichester District. by Dr
Streams, Ditches and Wetlands in the Chichester District. By Dr. Carolyn Cobbold, BSc Mech Eng., FRSA Richard C J Pratt, BA(Hons), PGCE, MSc (Arch), FRGS Despite the ‘duty of cooperation’ set out in the National Planning Policy Framework1, there is mounting evidence that aspects of the failure to deliver actual cooperation have been overlooked in the recent White Paper2. Within the subregion surrounding the Solent, it is increasingly apparent that the development pressures are such that we risk losing sight of the natural features that underscore not only the attractiveness of the area but also the area’s natural health itself. This paper seeks to focus on the aquatic connections which maintain the sub-region’s biological health, connections which are currently threatened by overdevelopment. The waters of this sub-region sustain not only the viability of natural habitat but also the human economy of employment, tourism, recreation, leisure, and livelihoods. All are at risk. The paper is a plea for greater cooperation across the administrative boundaries of specifically the eastern Solent area. The paper is divided in the following way. 1. Highlands and Lowlands in our estimation of worth 2. The Flow of Water from Downs to Sea 3. Wetlands and Their Global Significance 4. Farmland and Fishing 5. 2011-2013: Medmerry Realignment Scheme 6. The Protection and Enhancement of Natural Capital in The Land ‘In Between’ 7. The Challenge to Species in The District’s Wildlife Corridors 8. Water Quality 9. Habitat Protection and Enhancement at the Sub-Regional Level 10. The policy restraints on the destruction of natural capital 11. -

2016 Field Trips Programme – Hampshire
2016 Field Trips Programme – Hampshire The Branch is grateful to the site owners/managers of the nature reserves visited for maintaining public access and safeguarding these very special places of wildlife interest. The grid reference given is for the parking location/meeting point. Sunday 1 MayMay,, mmmeetmeet at 10:30am Catherington Down (SU690143(SU690143),), site: Hampshire County Council Leader: Ashley Whitlock, Tel: 02392 731266; Mobile: 07752 182340 Directions: meet in Lovedean Lane. LLeaveeave the A3(M) at Junction 2, taking the B2149 towards HorndeanHorndean,,,, pass Morrison’s and keep ahead into Catherington LLane.ane. Turn left at Roads Hill and then right into Lovedean LaneLane;; parkpark in Lovedean LLane.ane. A chance to see a lesser known site in the MeoMeonn Valley with good floraflora and faunafauna.... Aiming to see: Brimstone, Orange Tip and other early butterflies and moths Saturday 7 MayMay,, mmmeetmeet at 10:30am Little Butser Hill (SU711200) Leader: Ashley Whitlock, Tel: 02392 731266; Mobile: 07752 182340 Directions: Butser Hill is well signposted from the A3(M) at Clanfield. Meet at Butser Hill car park (Pay & Display)Display).. A chance to visvisitit another area of Butser with a substantisubstantialal Duke of Burgundy colony, to see Skylarks and BuzBuzzards,zards, and take in spectacular views towards Ashford Hill and the MeMeonon Valley. Very steepsteep in places. BBringring Lunch as we will be visiting another part of Butser in the afternoon. Aiming to see: Duke of Burgundy, Dingy and Grizzled Skipper, Small Heath, early moths Saturday 14 MayMay,, mmmeetmeet at 10:30am Stockbridge Down (SU375346), site: National Trust Leader: Ashley Whitlock, Tel: 02392 731266; Mobile: 07752 182340 Directions: Stockbridge Down is 1 mile east of StockbridgeStockbridge and 6 miles west of Winchester on the B3049B3049. -

View Characterisation and Analysis
South Downs National Park: View Characterisation and Analysis Final Report Prepared by LUC on behalf of the South Downs National Park Authority November 2015 Project Title: 6298 SDNP View Characterisation and Analysis Client: South Downs National Park Authority Version Date Version Details Prepared by Checked by Approved by Director V1 12/8/15 Draft report R Knight, R R Knight K Ahern Swann V2 9/9/15 Final report R Knight, R R Knight K Ahern Swann V3 4/11/15 Minor changes to final R Knight, R R Knight K Ahern report Swann South Downs National Park: View Characterisation and Analysis Final Report Prepared by LUC on behalf of the South Downs National Park Authority November 2015 Planning & EIA LUC LONDON Offices also in: Land Use Consultants Ltd Registered in England Design 43 Chalton Street London Registered number: 2549296 Landscape Planning London Bristol Registered Office: Landscape Management NW1 1JD Glasgow 43 Chalton Street Ecology T +44 (0)20 7383 5784 Edinburgh London NW1 1JD Mapping & Visualisation [email protected] FS 566056 EMS 566057 LUC uses 100% recycled paper LUC BRISTOL 12th Floor Colston Tower Colston Street Bristol BS1 4XE T +44 (0)117 929 1997 [email protected] LUC GLASGOW 37 Otago Street Glasgow G12 8JJ T +44 (0)141 334 9595 [email protected] LUC EDINBURGH 28 Stafford Street Edinburgh EH3 7BD T +44 (0)131 202 1616 [email protected] Contents 1 Introduction 1 Background to the study 1 Aims and purpose 1 Outputs and uses 1 2 View patterns, representative views and visual sensitivity 4 Introduction 4 View