Effector Memory T Helper Cells Secrete IFN-Γ Upon Stimulation with Cytokines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Correlation of Cytokine Level with the Severity of Severe Fever With
Liu et al. Virology Journal (2017) 14:6 DOI 10.1186/s12985-016-0677-1 RESEARCH Open Access Correlation of cytokine level with the severity of severe fever with thrombocytopenia syndrome Miao-Miao Liu1, Xiao-Ying Lei1, Hao Yu2, Jian-zhi Zhang3 and Xue-jie Yu1,4* Abstract Background: Severe fever with thrombocytopenia syndrome (SFTS) was an emerging hemorrhagic fever that was caused by a tick-borne bunyavirus, SFTSV. Although SFTSV nonstructural protein can inhibit type I interferon (IFN-I) production Ex Vivo and IFN-I played key role in resistance SFTSV infection in animal model, the role of IFN-I in patients is not investigated. Methods: We have assayed the concentration of IFN-α, a subtype of IFN-I as well as other cytokines in the sera of SFTS patients and the healthy population with CBA (Cytometric bead array) assay. Results: The results showed that IFN-α, tumor necrosis factor (TNF-α), granulocyte colony-stimulating factor (G-CSF), interferon-γ (IFN-γ), macrophage inflammatory protein (MIP-1α), interleukin-6 (IL-6), IL-10, interferon-inducible protein (IP-10), monocyte chemoattractant protein (MCP-1) were significantly higher in SFTS patients than in healthy persons (p < 0.05); the concentrations of IFN-α, IFN-γ, G-CSF, MIP-1α, IL-6, and IP-10 were significant higher in severe SFTS patients than in mild SFTS patients (p < 0.05). Conclusion: The concentration of IFN-α as well as other cytokines (IFN-γ, G-CSF, MIP-1α, IL-6, and IP-10) is correlated with the severity of SFTS, suggesting that type I interferon may not be significant in resistance SFTSV infection in humans and it may play an import role in cytokine storm. -

Influence of Infection and Inflammation on Biomarkers of Nutritional Status
A2.4 INFLUENCE OF INFECTION AND INFLAMMATION ON BIOMARKERS OF NUTRITIONAL STATUS A2.4 Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron David I. Thurnham1 and George P. McCabe2 1 Northern Ireland Centre for Food and Health, University of Ulster, Coleraine, United Kingdom of Great Britain and Northern Ireland 2 Statistics Department, Purdue University, West Lafayette, Indiana, United States of America Corresponding author: David I. Thurnham; [email protected] Suggested citation: Thurnham DI, McCabe GP. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization. Report: Priorities in the assessment of vitamin A and iron status in populations, Panama City, Panama, 15–17 September 2010. Geneva, World Health Organization, 2012. Abstract n Many plasma nutrients are influenced by infection or tissue damage. These effects may be passive and the result of changes in blood volume and capillary permeability. They may also be the direct effect of metabolic alterations that depress or increase the concentration of a nutrient or metabolite in the plasma. Where the nutrient or metabolite is a nutritional biomarker as in the case of plasma retinol, a depression in retinol concentrations will result in an overestimate of vitamin A deficiency. In contrast, where the biomarker is increased due to infection as in the case of plasma ferritin concentrations, inflammation will result in an underestimate of iron deficiency. Infection and tissue damage can be recognized by their clinical effects on the body but, unfortunately, subclinical infection or inflammation can only be recognized by measur- ing inflammation biomarkers in the blood. -

The Gut Microbiota and Inflammation
International Journal of Environmental Research and Public Health Review The Gut Microbiota and Inflammation: An Overview 1, 2 1, 1, , Zahraa Al Bander *, Marloes Dekker Nitert , Aya Mousa y and Negar Naderpoor * y 1 Monash Centre for Health Research and Implementation, School of Public Health and Preventive Medicine, Monash University, Melbourne 3168, Australia; [email protected] 2 School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane 4072, Australia; [email protected] * Correspondence: [email protected] (Z.A.B.); [email protected] (N.N.); Tel.: +61-38-572-2896 (N.N.) These authors contributed equally to this work. y Received: 10 September 2020; Accepted: 15 October 2020; Published: 19 October 2020 Abstract: The gut microbiota encompasses a diverse community of bacteria that carry out various functions influencing the overall health of the host. These comprise nutrient metabolism, immune system regulation and natural defence against infection. The presence of certain bacteria is associated with inflammatory molecules that may bring about inflammation in various body tissues. Inflammation underlies many chronic multisystem conditions including obesity, atherosclerosis, type 2 diabetes mellitus and inflammatory bowel disease. Inflammation may be triggered by structural components of the bacteria which can result in a cascade of inflammatory pathways involving interleukins and other cytokines. Similarly, by-products of metabolic processes in bacteria, including some short-chain fatty acids, can play a role in inhibiting inflammatory processes. In this review, we aimed to provide an overview of the relationship between the gut microbiota and inflammatory molecules and to highlight relevant knowledge gaps in this field. -

MST1 Is a Key Regulator of Beta Cell Apoptosis and Dysfunction in Diabetes
ARTICLES MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes Amin Ardestani1, Federico Paroni1,6, Zahra Azizi1,6, Supreet Kaur1,6, Vrushali Khobragade1, Ting Yuan1, Thomas Frogne2, Wufan Tao3, Jose Oberholzer4, Francois Pattou5, Julie Kerr Conte5 & Kathrin Maedler1 Apoptotic cell death is a hallmark of the loss of insulin-producing beta cells in all forms of diabetes mellitus. Current treatments fail to halt the decline in functional beta cell mass, and strategies to prevent beta cell apoptosis and dysfunction are urgently needed. Here, we identified mammalian sterile 20–like kinase-1 (MST1) as a critical regulator of apoptotic beta cell death and function. Under diabetogenic conditions, MST1 was strongly activated in beta cells in human and mouse islets and specifically induced the mitochondrial-dependent pathway of apoptosis through upregulation of the BCL-2 homology-3 (BH3)-only protein BIM. MST1 directly phosphorylated the beta cell transcription factor PDX1 at T11, resulting in the latter’s ubiquitination and degradation and thus in impaired insulin secretion. MST1 deficiency completely restored normoglycemia, beta cell function and survival in vitro and in vivo. We show MST1 as a proapoptotic kinase and key mediator of apoptotic signaling and beta cell dysfunction and suggest that it may serve as target for the development of new therapies for diabetes. Pancreatic beta cell death is the fundamental cause of type 1 diabetes of events, which makes the initiation of beta cell death complex and (T1D) and a contributing factor to the reduced beta cell mass in type 2 its blockade difficult to successfully achieve in vivo. -

Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications
International Journal of Molecular Sciences Review Physical Exercise and Myokines: Relationships with Sarcopenia and Cardiovascular Complications Sandra Maria Barbalho 1,2,3,* , Uri Adrian Prync Flato 1,2 , Ricardo José Tofano 1,2, Ricardo de Alvares Goulart 1, Elen Landgraf Guiguer 1,2,3 , Cláudia Rucco P. Detregiachi 1 , Daniela Vieira Buchaim 1,4, Adriano Cressoni Araújo 1,2 , Rogério Leone Buchaim 1,5, Fábio Tadeu Rodrigues Reina 1, Piero Biteli 1, Daniela O. B. Rodrigues Reina 1 and Marcelo Dib Bechara 2 1 Postgraduate Program in Structural and Functional Interactions in Rehabilitation, University of Marilia (UNIMAR), Avenue Hygino Muzzy Filho, 1001, Marília 17525-902, São Paulo, Brazil; urifl[email protected] (U.A.P.F.); [email protected] (R.J.T.); [email protected] (R.d.A.G.); [email protected] (E.L.G.); [email protected] (C.R.P.D.); [email protected] (D.V.B.); [email protected] (A.C.A.); [email protected] (R.L.B.); [email protected] (F.T.R.R.); [email protected] (P.B.); [email protected] (D.O.B.R.R.) 2 School of Medicine, University of Marília (UNIMAR), Avenida Higino Muzzi Filho, 1001, Marília 17506-000, São Paulo, Brazil; [email protected] 3 Department of Biochemistry and Nutrition, Food Technology School, Marília 17525-902, São Paulo, Brazil 4 Medical School, University Center of Adamantina (UniFAI), Adamantina 17800-000, São Paulo, Brazil 5 Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo (FOB–USP), Alameda Doutor Octávio Pinheiro Brisolla, 9-75, Bauru 17012901, São Paulo, Brazil * Correspondence: [email protected]; Tel.: +55-14-99655-3190 Received: 6 May 2020; Accepted: 19 May 2020; Published: 20 May 2020 Abstract: Skeletal muscle is capable of secreting different factors in order to communicate with other tissues. -

Innate Immunity and Inflammation
ISBTc ‐ Primer on Tumor Immunology and Biological Therapy of Cancer InnateInnate ImmunityImmunity andand InflammationInflammation WillemWillem Overwijk,Overwijk, Ph.D.Ph.D. MDMD AndersonAnderson CancerCancer CenterCenter CenterCenter forfor CancerCancer ImmunologyImmunology ResearchResearch Houston,Houston, TXTX www.allthingsbeautiful.com InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. Adapted from Merriam‐Webster Medical Dictionary • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. • Inflammation: a local response to tissue injury – Rubor (redness) – Calor (heat) – Dolor (pain) – Tumor (swelling) Adapted from Merriam‐Webster Medical Dictionary ““InnateInnate ImmunityImmunity”” andand ““InflammationInflammation”” areare vaguevague termsterms •• SpecificSpecific cellcell typestypes andand moleculesmolecules orchestrateorchestrate specificspecific typestypes ofof inflammationinflammation -

Effect of Exercise Intensity on Cell-Mediated Immunity
sports Perspective Effect of Exercise Intensity on Cell-Mediated Immunity Katsuhiko Suzuki 1,* and Harumi Hayashida 2 1 Faculty of Sport Sciences, Waseda University, 2-579-15 Mikajima, Tokorozawa 359-1192, Japan 2 Faculty of Culture and Sport Policy, Toin University of Yokohama, 1614 Kurogane-cho, Aoba-ku, Yokohama 225-8503, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-4-2947-6898 Abstract: Moderate-intensity exercise is considered to enhance immune function and to be useful for preventing acute upper respiratory infections and similar conditions. Many people practice low- intensity short-duration exercise with the expectation of a beneficial effect on immunocompetency. However, it is difficult to affirm the existence of definite evidence of such a benefit. In this article, we discuss the effects of low-intensity short-duration exercise on cell-mediated immunity, and contrast them to the effects of high-intensity and long-duration exercise. Whereas high-intensity exercise induces inflammation and reduces cell-mediated immune system function, low-intensity exercise does not appear to have a large effect on either inflammation or cell-mediated immune function. Low-intensity exercises such as walking and yoga, which are helpful to relieve stress, cannot be considered as harmful to the immune system. Although yoga was shown to impose fewer restrictions on breathing and physical strain, the evidence that yoga enhances cell-mediated immunity remains insufficient. Therefore, further studies are needed to examine the exercise mode that may be most effective for improvement of immune functions. Keywords: exercise; walking; yoga; cellular immune system; cytokines; inflammation 1. -

Antigen-Specific Induced Foxp3 Regulatory T Cells Are Generated
Antigen-specific induced Foxp3+ regulatory T cells are generated following CD40/CD154 blockade Ivana R. Ferrer, Maylene E. Wagener, Minqing Song, Allan D. Kirk, Christian P. Larsen, and Mandy L. Ford1 Emory Transplant Center and Department of Surgery, Emory University, Atlanta, GA 30322 Edited by James P. Allison, Memorial Sloan-Kettering Cancer Center, New York, NY, and approved November 4, 2011 (received for review April 7, 2011) Blockade of the CD40/CD154 pathway potently attenuates T-cell in vivo accumulation of Treg and deletion of graft-specific ef- responses in models of autoimmunity, inflammation, and trans- fectors after CD40/CD154 blockade has not been investigated. plantation. Indeed, CD40 pathway blockade remains one of the To address these questions, we used an ovalbumin (OVA)- most powerful methods of prolonging graft survival in models of expressing transgenic mouse model (20), which allowed us to di- + + transplantation. But despite this effectiveness, the cellular and rectly track both donor-reactive CD4 and CD8 T-cell responses molecular mechanisms underlying the protective effects of CD40 simultaneously over time. Although previous studies have used pathway blockade are incompletely understood. Furthermore, the transgenic models to assess the degree of T-cell deletion after CD154/CD40 blockade (16, 21), none has systematically inter- relative contributions of deletion, anergy, and regulation have not + been measured in a model in which donor-reactive CD4+ and CD8+ rogated the impact of anti-CD154 on the kinetics of both CD4 and CD8+ T-cell deletion and acquisition of effector function. T-cell responses can be assessed simultaneously. To investigate the fi Our results indicated that although CD40/CD154 blockade alone impact of CD40/CD154 pathway blockade on graft-speci c T-cell delayed donor-reactive CD4+ and CD8+ T-cell expansion and responses, a transgenic mouse model was used in which recipients differentiation, the addition of DST was required for antigen- fi + + containing ovalbumin-speci cCD4 and CD8 TCR transgenic T specific T-cell deletion. -

An EANM Procedural Guideline
European Journal of Nuclear Medicine and Molecular Imaging https://doi.org/10.1007/s00259-018-4052-x GUIDELINES Clinical indications, image acquisition and data interpretation for white blood cells and anti-granulocyte monoclonal antibody scintigraphy: an EANM procedural guideline A. Signore1 & F. Jamar2 & O. Israel3 & J. Buscombe4 & J. Martin-Comin5 & E. Lazzeri6 Received: 27 April 2018 /Accepted: 6 May 2018 # The Author(s) 2018 Abstract Introduction Radiolabelled autologous white blood cells (WBC) scintigraphy is being standardized all over the world to ensure high quality, specificity and reproducibility. Similarly, in many European countries radiolabelled anti-granulocyte antibodies (anti-G-mAb) are used instead of WBC with high diagnostic accuracy. The EANM Inflammation & Infection Committee is deeply involved in this process of standardization as a primary goal of the group. Aim The main aim of this guideline is to support and promote good clinical practice despite the complex environment of a national health care system with its ethical, economic and legal aspects that must also be taken into consideration. Method After the standardization of the WBC labelling procedure (already published), a group of experts from the EANM Infection & Inflammation Committee developed and validated these guidelines based on published evidences. Results Here we describe image acquisition protocols, image display procedures and image analyses as well as image interpre- tation criteria for the use of radiolabelled WBC and monoclonal antigranulocyte antibodies. Clinical application for WBC and anti-G-mAb scintigraphy is also described. Conclusions These guidelines should be applied by all nuclear medicine centers in favor of a highly reproducible standardized practice. Keywords Infection . -
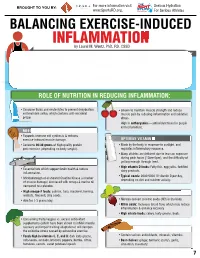
INFLAMMATION MICRONUTRIENTS SOURCES FUNCTION by Laurel M
For more information visit Serious Hydration For more information visit Serious Hydration BROUGHT TO YOU BY: BROUGHT TO YOU BY: For more information visit Serious Hydration www.SportsRD.org. For Serious Athletes www.SportsRD.org. For Serious Athletes www.SportsRD.org. NASA DEVELOPED For Serious Athletes NUTRITIONAL SUPPORT FOR INJURY BALANCING EXERCISE-INDUCED RECOVERY AND RETURN-TO-PLAY INFLAMMATION MICRONUTRIENTS SOURCES FUNCTION by Laurel M. Wentz, PhD, RD, CSSD Vitamin C Citrus fruit, red and green peppers, Antioxidant, wound healing, tissue repair, EXERCISE-INDUCED INFLAMMATION IS THE BODY’S RESPONSE TO INJURY DUE TO INTENSE PHYSICAL ACTIVITY cantaloupe immune function • The immune system response causes redness, swelling, pain. Vitamin A Sweet potato, spinach, carrots, Cell growth and development, • Acute inflammation is a normal response to high-intensity exercise, but prolonged (chronic) tomatoes immune function inflammation is a sustained response that affects the entire body. Vitamin D Sun exposure, oily fish, Promotes calcium absorption and bone health • Prolonged Inflammation: dairy products, fortified foods 1. Causes fatigue, muscle damage and soreness. 2. Limits muscle growth and training progression and increases muscle loss. Calcium Low-fat milk, fortified non-dairy milk, Supports skeletal structure and function 3. Modulating prolonged inflammation may enhance recovery & reduce soreness. low-fat Greek yogurt, cheese, broccoli, kale, fortified orange juice Magnesium Almonds, sesame and sunflower Nucleic acid and protein -

Human CD154 (CD40 Ligand) Recombinant Protein Catalog Number: 14-8502 Also Known As:CD40L, CD40-L RUO: for Research Use Only
Human CD154 (CD40 Ligand) Recombinant Protein Catalog Number: 14-8502 Also Known As:CD40L, CD40-L RUO: For Research Use Only Product Information Contents: Human CD154 (CD40 Ligand) Recombinant Protein Formulation: Sterile liquid: phosphate buffered saline, pH 7.2, Catalog Number: 14-8502 1.0% BSA. 0.22 µm filtered. Handling Conditions: For best recovery, quick-spin vial prior to Temperature Limitation: Store at less than or equal to -70°C. opening. Use in a sterile environment Batch Code: Refer to Vial Source: E. coli Use By: Refer to Vial Purity: Greater than 98%, as determined by SDS-PAGE Endotoxin Level: Less than 0.01 ng/ug cytokine as determined by the LAL assay. Bioactivity: The ED50 measured in a T-47D cell line proliferation assay is typically 40 ng/ml, corresponding to a specific activity of approximately 2.5 x104 Units/mg. Description CD40 ligand, (CD40L, also known as CD154, TRAP or gp39) is a membrane glycoprotein expressed on activated CD4+ T-cells, NK cells, mast cells, basophils and eosinophils. The CD40-CD40L interaction stimulates B cell immune response which includes cell surface antigen expression, cell cycle activation, Ig isotype switching, Ig secretion and memory generation. The CD40-CD40L interaction also plays important roles in monocyte and dendritic cell activation, T-cell co-stimulation and cytokine production. It has been reported that the CD40-CD40L interaction is involved in the pathogenesis of amyloid pathology in Alzheimer disease. Recombinant Human CD40L produced in E.Coli is a non-glycosylated, polypeptide containing 149 amino acids and having a molecular mass of 16 kDa. -

A Role of Inflammation and Immunity in Essential Hypertension—Modeled and Analyzed Using Petri Nets
International Journal of Molecular Sciences Article A Role of Inflammation and Immunity in Essential Hypertension—Modeled and Analyzed Using Petri Nets Dorota Formanowicz 1 , Agnieszka Rybarczyk 2,3 , Marcin Radom 2,3 and Piotr Formanowicz 2,3,* 1 Department of Clinical Biochemistry and Laboratory Medicine, Poznan University of Medical Sciences, 60-806 Poznan, Poland; [email protected] 2 Institute of Computing Science, Poznan University of Technology, 60-965 Poznan, Poland; [email protected] (A.R.); [email protected] (M.R.) 3 Institute of Bioorganic Chemistry, Polish Academy of Sciences, 61-704 Poznan, Poland * Correspondence: [email protected] Received: 15 April 2020; Accepted: 5 May 2020; Published: 9 May 2020 Abstract: Recent studies have shown that the innate and adaptive immune system, together with low-grade inflammation, may play an important role in essential hypertension. In this work, to verify the importance of selected factors for the development of essential hypertension, we created a Petri net-based model and analyzed it. The analysis was based mainly on t-invariants, knockouts of selected fragments of the net and its simulations. The blockade of the renin-angiotensin (RAA) system revealed that the most significant effect on the emergence of essential hypertension has RAA activation. This blockade affects: (1) the formation of angiotensin II, (2) inflammatory process (by influencing C-reactive protein (CRP)), (3) the initiation of blood coagulation, (4) bradykinin generation via the kallikrein-kinin system, (5) activation of lymphocytes in hypertension, (6) the participation of TNF alpha in the activation of the acute phase response, and (7) activation of NADPH oxidase—a key enzyme of oxidative stress.