The Current State of Biodegradable Self-Expanding Stents in Interventional Gastrointestinal and Pancreatobiliary Endoscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Esophageal Stents After Anastomotic Leakage in Surgery for Gastric Adenocarcinoma Case Report and Review of the Literature
ISSN: 2574-1241 Volume 5- Issue 4: 2018 DOI: 10.26717/BJSTR.2018.06.001391 Fernando Mendoza-Moreno. Biomed J Sci & Tech Res Case Report Open Access Use of Esophageal Stents After Anastomotic Leakage in Surgery for Gastric Adenocarcinoma Case Report and Review of the Literature Mendoza-Moreno F*1, Díez-Gago MR2, Mínguez-García J1, Enjuto-Martínez DT1,Tallón-Iglesias B1, Solana-Maoño M1 and Argüello-de-Andrés JM1 1Department of General and Digestive Surgery,Sanitas La Moraleja Teaching Hospital, Spain 2Department of Emergency, Príncipe de Asturias Teaching Hospital, Spain Received: July 4, 2018; Published: July 12, 2018 *Corresponding author: Fernando Mendoza-Moreno, Department of General and Digestive Surgery, Sanitas La Moraleja Teaching Hospital, Madrid, Spain Abstract Introduction: Radical gastrectomy is the treatment of choice for the treatment of gastric cancer located in the upper third of stomach or in case of diffuse histology or cells in a signet ring. The worst complication after a radical gastrectomy is the leakage of the esophago-jejunal anastomosis, since it considerably increases the morbidity and mortality of the patient. Case Report: Wedescribe our experience after performing a radical gastrectomy for gastric adenocarcinoma in a patient who developed a leakage of the esophago-jejunal anastomosis in the postoperative period. Although he was reoperated, performing reinforcement of the anastomosis and making a feeding jejunostomy, the dehiscence progressed in the following days until it became almost complete. Then, we proceeded to place a digestive endoprosthesis through gastroscopy with good results, allowing the entire defect to heal and being able to be removed without incidents after 8 weeks. -

Stents for the Gastrointestinal Tract and Nutritional Implications
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #46 Carol Rees Parrish, R.D., M.S., Series Editor Stents for the Gastrointestinal Tract and Nutritional Implications Michelle Loch Michel Kahaleh Endoscopic stenting of many sites along the gastrointestinal tract is used successfully for palliation of malignant or benign obstructions. These obstructions may be the result of primary gastrointestinal tumors invading the lumen, tumors of another primary site causing external compression or in some instance benign diseases secondary to various inflammatory processes. Stenting of the gastrointestinal tract has been commonly per- formed either by interventional radiologists with the use of fluoroscopy, or by gas- troenterologists endoscopically, with or without fluoroscopic guidance. Their efficacy can be measured by resolution of obstruction or symptom improvement. The current literature shows that endoscopic stenting have acceptable success and complication rates and might be considered as first-line therapy in centers offering expertise in inter- ventional endoscopy. The techniques, efficacy and complication of stenting will be dis- cussed. Nutritional guidelines will also be provided based on our institutions practice. INTRODUCTION most current literature (Table 1) (4–9). Common causes ndoscopy within the last two decades has encom- of stent requirement to preserve nutritional status passed many interventional procedures allowing include esophageal, duodenal, biliary and colonic Ethe treatment of multiple conditions of the upper obstruction; -

Long-Term Outcomes and Complications of Metallic Stents for Malignant Esophageal Stenoses
Kobe J. Med. Sci., Vol. 49, No. 6, pp. 133-142, 2003 Long-term Outcomes and Complications of Metallic Stents for Malignant Esophageal Stenoses RYOTA KAWASAKI1, AKIRA SANO2, and SHINICHI MATSUMOTO2 Department of Radiology, Kobe University Graduate School of Medicine, Kobe1, Department of Radiology, Tenri Hospital, Tenri, Nara2 Received 13 January 2004/ Accepted 6 February 2004 Key words: Esophagus; Malignant Stenoses; Ultraflex Stents; Outcomes; Complications Thirty patients with malignant esophageal stenosis underwent Ultraflex esophageal stent deployment and were followed up for a maximum of 29 months from June 1995 to August 2001 in Tenri Hospital. Twelve stents were in the upper esophagus, and nine each in the middle and lower esophagus. The procedures were successful and dysphagia scores improved from 2.9 to 0.7. Major complications such as esophagorespiratory fistula, hematemesis, or airway compression occurred in 9 patients, more often in the upper esophagus than in other parts of the esophagus, with no statistical difference. There was a significant difference in the onset of major complications between the upper and middle esophagus, as well as between the upper and middle-lower esophagus (p<0.05), but no difference in mean survival time between locations, or patients with or without major complications. These results demonstrate that esophageal stent deployment is effective for relieving dysphagia and associating malnutrition. But major complications may occur in the upper esophagus more often and earlier than in other parts. Metallic stents are effective in relieving those patients with malignant esophageal stenosis from their suffering from dysphagia and malnutrition. On the other hand, several serious complications after the stent deployment or during the long-term follow up have been described in the literature, which include massive bleeding, tracheal compression, esophagorespiratory fistula, and esophageal compression1-10). -

Tracheoesophageal Fistula(TEF), Tracheostomy, Nasogastric Tube, Esophageal Stent, Trichloroacetic Acid (TCA)
hf RACI PHAGEAL FISTULA: ON IN MANAGEMENT Surinder K. Singhal,* Ramandeep S.Virk, ** Arjun Dass,*** Biinaljit Singh Sandhu**** Key words: Tracheoesophageal fistula(TEF), Tracheostomy, Nasogastric tube, esophageal stent, trichloroacetic Acid (TCA). INTRODUCTION: & vomiting after taking food. The patient was a known diabetic and hypertensive on regular treatment. She had undergone a Tracheoesophageal fistula (TEF) is a communication between the medical termination of pregnancy and developed a faecal fistula. esophagus and trachea which can be congenital or acquired. She was operated for the fecal fistula and a colostomy was also About 80% of the acquired tracheoesophageal fistulae are done but on the second post operative day she had cardiac malignant and rest non-malignant' . arrest. She was intubated and revived. Later she underwent a Nonmalignant fistulae are usually due to trauma which can be tracheostomy and remained on ventilatory support for two iatrogenic, internal or external. latrogenic fistulae may occur weeks. She was gradually weaned off the ventilator and on 19` 1 following mechanical ventilation or as a complication of day of tracheostomy she was decanulated and discharged. tracheostomy. Internal trauma may be due to cuffed endotracheal She came back after a week with the complaints of violent cough, tube or nasogastric tubes or a combination of both. It may be regurgitation and vomiting after meals. Systemic examination external trauma from penetrating foreign bodies, open or closed was within normal limits. Colostomy bag was in situ. Local aero digestive tract injuries' .There are many risk factors examination revealed a tracheostomy scar. Oral cavity and associated with post intubation tracheoesophageal fistula like Oropharynx were unremarkable. -

Biodegradable Esophageal Stents for the Treatment of Refractory Benign Esophageal Strictures
INVITED REVIEW Annals of Gastroenterology (2020) 33, 1-8 Biodegradable esophageal stents for the treatment of refractory benign esophageal strictures Paraskevas Gkolfakisa, Peter D. Siersemab, Georgios Tziatziosc, Konstantinos Triantafyllouc, Ioannis S. Papanikolaouc Erasme University Hospital, Université Libre de Bruxelles, Brussels, Belgium; Radboud University Medical Center, Nijmegen, The Netherlands; “Attikon” University General Hospital, Medical School, National and Kapodistrian University of Athens, Greece Abstract This review attempts to present the available evidence regarding the use of biodegradable stents in refractory benign esophageal strictures, especially highlighting their impact on clinical success and complications. A comprehensive literature search was conducted in PubMed, using the terms “biodegradable” and “benign”; evidence from cohort and comparative studies, as well as data from one pooled analysis and one meta-analysis are presented. In summary, the results from these studies indicate that the effectiveness of biodegradable stents ranges from more than one third to a quarter of cases, fairly similar to other types of stents used for the same indication. However, their implementation may reduce the need for re-intervention during follow up. Biodegradable stents also seem to reduce the need for additional types of endoscopic therapeutic modalities, mostly balloon or bougie dilations. Results from pooled data are consistent, showing moderate efficacy along with a higher complication rate. Nonetheless, the validity of these results is questionable, given the heterogeneity of the studies included. Finally, adverse events may occur at a higher rate but are most often minor. The lack of high-quality studies with sufficient patient numbers mandates further studies, preferably randomized, to elucidate the exact role of biodegradable stents in the treatment of refractory benign esophageal strictures. -

Redalyc.Esophageal Metalic Stent Migration. Case Report of A
Acta Gastroenterológica Latinoamericana ISSN: 0300-9033 [email protected] Sociedad Argentina de Gastroenterología Argentina Rubio Mainardi, María Soledad; Alcaraz, Álvaro; Patricia, Saleg; Romero, María Eugenia; Moser, Federico; Obeide, Lucio Ricardo Esophageal metalic stent migration. Case report of a dislodged stent retrieval Acta Gastroenterológica Latinoamericana, vol. 45, núm. 4, 2015, pp. 320-322 Sociedad Argentina de Gastroenterología Buenos Aires, Argentina Available in: http://www.redalyc.org/articulo.oa?id=199343433010 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative ♦CASO CLÍNICO Esophageal metalic stent migration. Case report of a dislodged stent retrieval María Soledad Rubio Mainardi, Álvaro Alcaraz, Saleg Patricia, María Eugenia Romero, Federico Moser, Lucio Ricardo Obeide Departamento de Cirugía General del Hospital Privado de Córdoba. Córdoba, Argentina. Acta Gastroenterol Latinoam 2015;45:320-322 Recibido: 29/03/2015 / Aprobado: 10/07/2015 / Publicado en www.actagastro.org el 30/12/2015 Summary ciente se recuperó sin complicaciones. Conclusión. Este caso Background. Metallic stent placing is the first choice in the ilustra que la técnica laparoscópica puede ser una forma opcio- treatment of malign or benign strictures of the esophagus. Stent nal para recuperar stents migrados en pacientes seleccionados. migration is a well-known complication of this procedure. We Palabras claves. Migración de stent, complicación de stent, report a case of stent migration in which surgical laparoscopic técnica laparoscópica, cáncer esofágico. intervention was used to retrieve it. Methods. -
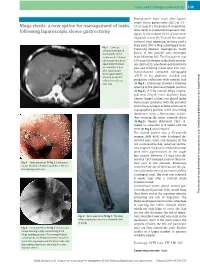
Mega Stents: a New Option for Management of Leaks Following Laparoscopic Sleeve Gastrectomy… Endoscopy 2014; 46: E49–E50 E50 Cases and Techniques Library (CTL)
Cases and Techniques Library (CTL) E49 Postoperative leaks occur after laparo- scopic sleeve gastrectomy (LSG) in 1% – Mega stents: a new option for management of leaks 3% of cases [1]. Placement of covered me- following laparoscopic sleeve gastrectomy tallic stents is an effective treatment strat- egy [2,3], but in about 16.9% of cases stent migration occurs [4]. To avoid the compli- cation of stent migration, we have used a large stent (Niti-S Mega esophageal stent; Fig. 1 Contrast- Taewoong Medical, Gyeonggi-do, South enhanced computed tomography of the Korea) in two patients who developed abdomen of a 50-year- leaks following LSG. The first patient was old woman who devel- a 50-year-old woman with a body mass in- oped abdominal pain dex (BMI) of 35, who developed abdominal and vomiting 2 days pain and vomiting 2 days after LSG. Con- after laparoscopic trast-enhanced computed tomography sleeve gastrectomy, (CECT) of her abdomen revealed two showing perigastric collections with con- perigastric collections with contrast leak trast leak. (●" Fig.1). Endoscopy showed a fistulous opening at the gastroesophageal junction (●" Fig.2). A fully covered Mega esopha- geal stent (length 23cm; diameter: body 24mm, flanges 32mm) was placed under fluoroscopic guidance with the proximal end in the esophagus and the distal end in suprapapillary position in the descending duodenum using a fluoroscopic marker, thus covering the entire stomach sleeve (●" Fig.3). Repeat abdominal CECT re- vealed no collection at 8 weeks and the stent (●" Fig.4) was removed. The second patient was a 45-year-old woman (BMI 48.4) who developed ab- dominal pain, fever, and dyspnea on the 3rd postoperative day, requiring ventila- tory support. -

Development and Implementation of a New Nitinol Stent Design for Managing Benign Stenoses and Fistulas of the Digestive Tract
Original articles Development and Implementation of a New Nitinol Stent Design for Managing Benign Stenoses and Fistulas of the Digestive Tract Rodrigo Castaño, MD,1 Oscar Álvarez, MD,2 Jorge Lopera,3 Mario H. Ruiz, MD,4 Andrés Rojas, MD,5 Alejandra Álvarez,6 Luis Miguel Ruiz,6 David Restrepo.7 1 Gastrointestinal Surgery and Endoscopy. Abstract Chief of Postgraduate General Surgery (UPB), Gastrohepatology Group at the University of Background: Benign stenoses, digestive tract ruptures and fistulas are conditions that endanger life and Antioquia, Institute of Clinical Oncology of the are often treated surgically. Recently, the placement of partially or fully covered metal stents has emerged Americas in Medellin, Colombia. rcastanoll@ as a minimally invasive treatment option. This article looks at a new design for stents to determine its clinical hotmail.com 2 Gastroenterologist at Texas Valley Coastal Bend effectiveness. The new stent is a completely covered nitinol stent for treatment of gastrointestinal perforations Veterans Administration Hospital and Clinical Assistant and anastomotic leaks. This article places special emphasis on evaluating reactive hyperplasia. Professor at the University of Texas Health Science Methods: Fifteen had the new completely covered self-expanding nitinol stent placed for treatment of Center at San Antonio in San Antonio, Texas USA 3 Interventional Radiologist at the University of Texas benign esophageal perforations, anastomotic leaks, and stenoses following upper or lower gastrointestinal in the United States. surgery during 2012 and 2013. The stents are 20 mm in diameter in the middle and 28 mm in diameter at the 4 General Surgeon at the Hospital Pablo Tobón Uribe in proximal end. -

Adverse Events of Upper GI Endoscopy
GUIDELINE Adverse events of upper GI endoscopy This is one of a series of statements discussing the use of lications rely on self-reporting, and most reported data GI endoscopy in common clinical situations. The Stan- collected only from the immediate periprocedure period, dards of Practice Committee of the American Society for thus the rate of late adverse events and mortality may be Gastrointestinal Endoscopy (ASGE) prepared this text. underestimated.8,9 Major adverse events related to diag- In preparing this document, a search of the medical liter- nostic UGI endoscopy are rare and include cardiopulmo- ature was performed by using PubMed. Additional refer- nary adverse events, infection, perforation, and bleeding. ences were obtained from the bibliographies of the identi- Adverse events of ERCP and EUS are discussed in separate fied articles and from recommendations of expert ASGE documents.10,11 consultants. When few or no data exist from well-designed prospective trials, emphasis is given to results of large series and reports from recognized experts. This document is ADVERSE EVENTS ASSOCIATED WITH based on a critical review of the available data and expert DIAGNOSTIC UGI ENDOSCOPY consensus at the time that the document was drafted. Further controlled clinical studies may be needed to clar- Cardiopulmonary adverse events ify aspects of this document. This document may be re- Most UGI procedures in the United States and Europe vised as necessary to account for changes in technology, are performed with patients under sedation (moderate or 12 new data, or other aspects of clinical practice. deep). Cardiopulmonary adverse events related to seda- This document is intended to be an educational device tion and analgesia account for as much as 60% of UGI 1-4,7 to provide information that may assist endoscopists in endoscopy adverse events. -

Diagnosis and Management of Iatrogenic Endoscopic Perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement
Guideline Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement Authors Gregorios A. Paspatis1, Jean-Marc Dumonceau2, Marc Barthet3, Søren Meisner4, Alessandro Repici5, Brian P. Saunders6, Antonios Vezakis7, Jean Michel Gonzalez3, Stine Ydegaard Turino4, Zacharias P. Tsiamoulos6, Paul Fockens8, Cesare Hassan9 Institutions Institutions are listed at the end of article. Bibliography This Position Paper is an official statement of the European Society of Gastrointestinal Endoscopy DOI http://dx.doi.org/ (ESGE). It addresses the diagnosis and management of iatrogenic perforation occurring during diag- 10.1055/s-0034-1377531 nostic or therapeutic digestive endoscopic procedures. Published online: 2014 Endoscopy © Georg Thieme Verlag KG Main recommendations 4 ESGE recommends that endoscopic closure Stuttgart · New York 1 ESGE recommends that each center imple- should be considered depending on the type of ISSN 0013-726X ments a written policy regarding the manage- perforation, its size, and the endoscopist exper- ment of iatrogenic perforation, including the de- tise available at the center. A switch to carbon Corresponding author Gregorios A. Paspatis, MD finition of procedures that carry a high risk of dioxide insufflation, the diversion of luminal Gastroenterology Department this complication. This policy should be shared content, and decompression of tension pneu- Benizelion General Hospital with the radiologists and surgeons at each cen- moperitoneum or -

Effectiveness of a Novel Covered Stent Without External Thread Fixation for Anastomotic Leakage After Total Or Proximal Gastrectomy for Gastric Cancer
cancers Article Effectiveness of a Novel Covered Stent without External Thread Fixation for Anastomotic Leakage after Total or Proximal Gastrectomy for Gastric Cancer Young-Il Kim , Chan Gyoo Kim * , Jong Yeul Lee, Il Ju Choi, Bang Wool Eom, Hong Man Yoon, Keun Won Ryu and Young-Woo Kim Center for Gastric Cancer, National Cancer Center, 323 Ilsan-ro, Goyang 10408, Korea; [email protected] (Y.-I.K.); [email protected] (J.Y.L.); [email protected] (I.J.C.); [email protected] (B.W.E.); [email protected] (H.M.Y.); [email protected] (K.W.R.); [email protected] (Y.-W.K.) * Correspondence: [email protected]; Tel.: +82-31-920-1620 Simple Summary: A covered self-expandable metal stent using external fixation using silk thread (thread-fix stent) is an effective treatment for anastomotic leakage after esophago-gastric surgery. However, a thread-fix stent also entails long hospitalization and patient discomfort. This study found that the Niti-S Beta stent which does not need thread-fix was an effective treatment for anastomotic leakage after total or proximal gastrectomy for gastric cancer. Because patients who received the Nitis-S Beta stent had minimal discomfort, the stent maintenance was possible without hospitalization. Citation: Kim, Y.-I.; Kim, C.G.; Lee, Abstract: A thread-fix stent entails long hospitalization and patient discomfort. We aimed to evaluate J.Y.; Choi, I.J.; Eom, B.W.; Yoon, H.M.; the efficacy of a novel stent with silicone-covered outer double layers without external fixation (Beta Ryu, K.W.; Kim, Y.-W. -

The Channel a COOK News Publication Issue 3, 2008
The Channel A COOK NEWS PUBLICATION ISSUE 3, 2008 INSIDE THIS ISSUE HDFNA: AN IMPORTANT KEY 3 TO PRECISION COOK MEDICAL EnVIRONMENTAL ACTION PaYS 4 OFF IN SEVERAL GREEN WAYS GASTRIC MUCOSAL RESECTION 5 Commitment VIDEO CaSE CREATES INTEREST SUCCESSFUL ERCP/EUS 7 to Innovation WORKSHOP IN OSLO, NORwaY OKLAHOMA GASTROENTEROLO- giST DILATES EOSinOPhiLIC 8 Colonic and Duodenal stent system ESOPhagiTIS STRICTURE extends Cook Medical line of innovative COMING SOON: THE NEW ZILVER 635 SELF-EXPANDING 10 Controlled-Release Evolution devices BILIARY STENT SOFT ON TISSUE, STRONG 10 The recent launch of the Evolution® Controlled-Release Colonic and ON RESULTS Duodenal Stent Systems reflects Cook Medical’s continued commitment FUSION MARATHON 11 to pioneering important innovations in stent delivery and performance ANTI-REFLUX BILIARY STENT that can impact the quality of patient care. WHAt’s up DOC? 12 The stent system was created specifically for clinicians confronting malignant strictures, gastric outlet obstruction or creating a bridge to COOK IN THE NEWS 13 surgery. Providing excellent control and maneuverability, Evolution allows clinicians to precisely deliver a stent that provides better wall apposition, fully conforms to the natural curves of the anatomy and potentially NEWS FROM SIGNEA 14 reduces post-placement risks. BETH ISRAEL TEAM HONORED 15 COMMITMENT to INNOVation Continued on page 2 GI360 16 commitment to INNOVation Continued from page 1 Evolutionary Security What makes Evolution completely unique is the fact that it is the first and only stent delivery system that gives the clinician three important capabilities: the ability to deploy, recapture and/or reposition the stent. Its development is a major step forward in colonic and duodenal stenting, offering an innovative alternative to traditional deployment systems.