This Article Appeared in a Journal Published by Elsevier. the Attached Copy Is Furnished to the Author for Internal Non-Commerci
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Buteogallus Coronatus) in Argentina
SHORT COMMUNICATIONS J. Raptor Res. 54(2):166–171 Ó 2020 The Raptor Research Foundation, Inc. ELECTROCUTION ON POWER LINES IS AN IMPORTANT THREAT FOR THE ENDANGERED CHACO EAGLE (BUTEOGALLUS CORONATUS) IN ARGENTINA 1 JOSE´ H. SARASOLA Centro para el Estudio y Conservacio´n de Aves Rapaces en Argentina (CECARA). Universidad Nacional de La Pampa, Avda Uruguay 151, 6300 Santa Rosa, Argentina and Instituto de las Ciencias Ambientales y de la Tierra de La Pampa (INCITAP-CONICET), Avda Uruguay 151, 6300 Santa Rosa, La Pampa, Argentina MAXIMILIANO A. GALMES Centro para el Estudio y Conservacio´n de Aves Rapaces en Argentina (CECARA), Universidad Nacional de La Pampa, Avda Uruguay 151, 6300 Santa Rosa, Argentina and The Peregrine Fund, Boise, ID 83709 USA BRYAN D. WATTS Center for Conservation Biology, College of William & Mary, Williamsburg, VA 23187 USA and Virginia Commonwealth University, Williamsburg, VA 23284 USA ABSTRACT.—Electrocution is a widespread conservation problem for birds of prey that has received little attention in the Neotropics. Here we present electrocution records involving the endangered Chaco Eagle (Buteogallus coronatus) in central Argentina, and we provide information on the power pole structural characteristics associated with electrocutions. Nine Chaco Eagles were recorded electrocuted during the period 2012–2019 over an area of 9000 km2. Chaco Eagles were found electrocuted in association with five types of power poles, but more than half the electrocutions (55%) were on poles made of steel-reinforced concrete and with jumper wires above the crossarms. With the addition of four previous electrocution reports in this region during the same time period, the annual rate of Chaco Eagle electrocutions was similar to the rate of mortality by other human-related factors such as direct persecution. -

198 Pursuit and Capture of a Ring-Billed Gull by Bald
198 Florida Field Naturalist 28(4):198-200, 2000. PURSUIT AND CAPTURE OF A RING-BILLED GULL BY BALD EAGLES ANDREW W. KRATTER1 AND MARY K. HART2 1Florida Museum of Natural History, P.O. Box 117800, University of Florida Gainesville, Florida 3261; email: kratter@flmnh.ufl.edu 2Department of Fisheries and Aquatic Sciences, University of Florida Gainesville, Florida, 32611 Bald Eagles (Haliaeetus leucocephalus) are opportunistic hunters that employ a num- ber of techniques to capture a wide variety of prey (Bent 1937, Brown and Amadon 1968, Sherrod et al. 1976, McEwan and Hirth 1980). These eagles are known to occasionally pursue prey, including flying birds, in pairs or larger groups (McIlhenny 1932, Sherrod et al. 1976, Folk 1992). Here we report three Bald Eagles giving a prolonged chase of a Ring- billed Gull (Larus delawarensis), which resulted in the gull’s capture by one of the eagles. At approximately 1500 on 12 December 1998, we were kayaking east across Newnan’s Lake in eastern Alachua County, Florida, when we noticed a group of approximately 50 Ring-billed Gulls sitting on the water near the center of the lake. As we approached to approximately 100 m, the gulls lifted off when an adult Bald Eagle flew toward them at a height of about 75 m. The eagle immediately started to chase a first-winter individual. The chase was linear at first, but the gull evaded the faster flying eagle with sharp turns. Over the next three minutes, the eagle continued to chase the gull with rather slow stoops from heights of 25-100 m followed by short, fast linear pursuits. -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

Chromosome Painting in Three Species of Buteoninae: a Cytogenetic Signature Reinforces the Monophyly of South American Species
Chromosome Painting in Three Species of Buteoninae: A Cytogenetic Signature Reinforces the Monophyly of South American Species Edivaldo Herculano C. de Oliveira1,2,3*, Marcella Mergulha˜o Tagliarini4, Michelly S. dos Santos5, Patricia C. M. O’Brien3, Malcolm A. Ferguson-Smith3 1 Laborato´rio de Cultura de Tecidos e Citogene´tica, SAMAM, Instituto Evandro Chagas, Ananindeua, PA, Brazil, 2 Faculdade de Cieˆncias Exatas e Naturais, ICEN, Universidade Federal do Para´, Bele´m, PA, Brazil, 3 Cambridge Resource Centre for Comparative Genomics, Cambridge, United Kingdom, 4 Programa de Po´s Graduac¸a˜oem Neurocieˆncias e Biologia Celular, ICB, Universidade Federal do Para´, Bele´m, PA, Brazil, 5 PIBIC – Universidade Federal do Para´, Bele´m, PA, Brazil Abstract Buteoninae (Falconiformes, Accipitridae) consist of the widely distributed genus Buteo, and several closely related species in a group called ‘‘sub-buteonine hawks’’, such as Buteogallus, Parabuteo, Asturina, Leucopternis and Busarellus, with unsolved phylogenetic relationships. Diploid number ranges between 2n = 66 and 2n = 68. Only one species, L. albicollis had its karyotype analyzed by molecular cytogenetics. The aim of this study was to present chromosomal analysis of three species of Buteoninae: Rupornis magnirostris, Asturina nitida and Buteogallus meridionallis using fluorescence in situ hybridization (FISH) experiments with telomeric and rDNA probes, as well as whole chromosome probes derived from Gallus gallus and Leucopternis albicollis. The three species analyzed herein showed similar karyotypes, with 2n = 68. Telomeric probes showed some interstitial telomeric sequences, which could be resulted by fusion processes occurred in the chromosomal evolution of the group, including the one found in the tassociation GGA1p/GGA6. -

Field Identification of the Field Identification of the Field
TOPICS IN IDENTIFICATION he Solitary Eagle ( Harpyhaliaetus solitarius ) is a large raptor that is closely related and similar in adult and immature plum- Tages to the black-hawks in the genus Buteogallus (Lerner and Mindell 2005). It is a rare and very local resident in a variety of wet and dry forested hills and highlands from northern Argentina to northern Mexico (del Hoyo et al. 1994, Ferguson-Lees and Christie 2001). The species has been collected in Mexico not far from the Texas border (see Discussion, pp. 72 –73), so it is possible that it has occurred in the ABA Area. The handful of specimens and nest records of this eagle are from 700 to 2,000 meters above sea level (Brown and Amadon 1968). FFiieelldd IIddeennttiifificcaattiioonn ooff tthhee SSOOLLIITTTAAARRRYYY EEAAAGGGLLLEEE Nevertheless, sightings of this eagle are occasionally reported from lowland tropical rain forest, e.g., at Tikal, Guatemala (Beaver et al. 1991) and the Tuxtlas Mountains of south - William S. Clark ern Veracruz, Mexico (Winker et al. 1992). The species has been reported on some pro - 2301 South Whitehouse Circle fessional bird tours at such lowland sites as Palenque and the Usumicinta River in south - Harlingen, Texas 78550 ern Mexico. All of these accounts have relied on large size and gray coloration as the [email protected] field marks to distinguish the eagles from the much more abundant Common Black- Hawk ( Buteogallus anthracinus ) and Great Black-Hawk ( B. urubitinga ). H. Lee Jones Howell and Webb (1995) were skeptical and stated that most lowland records of the 4810 Park Newport, No. -

The Use of Green Plant Material in Bird Nests to Avoid Ectoparasites
July1984] ShortCommunications 615 the Spot-wingedFalconet may not benefitthe Monk HoY, G. 1980. Notas nidobio16gicasdel noroestear- Parakeet in such a manner. gentino. II. Physis (Buenos Aires), Secc. C, 39 We are grateful to A. G6mez Dur&n and J. C. Vera (96): 63-66. (INTA) for grantingus the useof the fieldwork areas, MACLEAN,G.L. 1973. The SociableWeaver, part 4: to N. Arguello and M. Nores for their assistancein predators, parasites and symbionts. Ostrich 44: the identification of food remains, and to J. Navarro 241-253. for his help in the field. This work wassupported by STRANECK,R., & G. VASINA. 1982. Unusual behav- a grant from the Subsecretariade Ciencia y Tecno- iour of the Spot-winged Falconet (Spiziapteryx logla (SUBCYT) of Argentina. circumcinctus).Raptor Res. 16: 25-26. LITERATURE CITED Received7 July 1983, accepted21 February1984. DEAN, A. 1971. Notes on Spiziapteryxcircumcinctus. Ibis 113: 101-102. The Use of Green Plant Material in Bird Nests to Avoid Ectoparasites PETER H. WIMBERGER 1 ZoologyDivision, Washington State Museum DB-10, Universityof Washington, Seattle,Washington 98105 USA Certain birds characteristicallyplace green plant causesnestling mortality in and nest desertion by material in their nests.This greenery is not part of birds (Webster 1944, Neff 1945, Fitch et al. 1946, Moss the nest structureproper but is placed haphazardly and Camin 1970, Feare 1976,Wheelwright and Boers- around the edges or inside the nest. The birds re- ma 1979).In general,the increasedmortality due to plenishthe spraysof greenmaterial, often daily, dur- ectoparasitesis causedby the loss of blood, which ing incubation and the nestling period (Brown and weakens the host, by viral disease, or by disease Amadon 1968, Beebe1976, pers. -

Spizaetus Boletin De La Red De Rapaces Neotropicales
SPIZAETUS BOLETIN DE LA RED DE RAPACES NEOTROPICALES NÚMERO 27 JUNIO 2019 BUTEO PLATYPTERUS EN COSTA RICA MILVAGO CHIMACHIMA EN COSTA RICA ASIO STYGIUS EN COLOMBIA BUTEO BRACHYURUS EN MÉXICO FALCO FEMORALIS EN MÉXICO SPIZAETUS BOLETIN DE LA RRN Número 27 © Junio 2019 Edición en Español, ISSN 2157-8966 Foto de la Portada: Juvenil de Gavilán Aludo (Buteo platypterus) en Costa Rica © Víctor Acosta-Chaves Traductores/Editores: Laura Andréa Lindenmeyer de Sousa & Marta Curti Diseño Gráfico: Marta Curti Spizaetus: Bolettin de la Red de Rapaces Neotropicales © Junio 2019 www.neotropicalraptors.org Este boletín puede ser reproducido, descargado y distribuido por fines no comerciales. Para volvera publicar cualquier artículo que figuran en este documento, por favor póngase en contacto con los autores correspondientes Contenido Singular comportamiento del caracara cabecigualdo (MILVAGO CHIMACHIMA: Falconidae) en el sur de Costa Rica José Manuel Mora & Estefanía González ............................................................................2 Nuevas localidades en Colombia del Búho Negruzco (ASIO STYGIUS) Elvis Felipe Quintero Quintero, Jeyson Sanabria-Mejía, Angélica Magaly Sogamoso Hernández & Sergio Chaparro-Herrera .................................................................................................................9 Un caso de agresión de BUTEO BRACHYURUS contra PSEUDASTUR ALBICOLLIS (Accipi- triformes) en el sur de Huimanguillo, Tabasco, México Saúl Sánchez Soto .......................................................................................................13 -

Trinidad & Tobago 2018 Species List
Trinidad Tobago Leader: Ernesto Carman Eagle-Eye Tours Nov 29 - Dec 9, 2018 Bird Species Seen/ Common Name Scientific Name Heard TINAMOUS 1 Little Tinamou Crypturellus soui H GUANS, CHACHALACAS, AND CURASSOWS 2 Trinidad Piping-Guan Aburria pipile S 3 Rufous-vented Chachalaca Ortalis ruficauda S FLAMINGOS 4 American Flamingo Phoenicopterus ruber S TROPICBIRDS 5 Red-billed Tropicbird Phaethon aethereus S FRIGATEBIRDS 6 Magnificent Frigatebird Fregata magnificens S BOOBIES AND GANNETS 7 Brown Booby Sula leucogaster S 8 Red-footed Booby Sula sula S CORMORANTS AND SHAGS 9 Neotropic Cormorant Phalacrocorax brasilianus S ANHINGAS 10 Anhinga Anhinga anhinga S PELICANS 11 Brown Pelican Pelecanus occidentalis S HERONS, EGRETS, AND BITTERNS 12 Pinnated Bittern Botaurus pinnatus S 13 Great Egret Ardea alba S 14 Little Egret Egretta garzetta S 15 Snowy Egret Egretta thula S 16 Little Blue Heron Egretta caerulea S 17 Tricolored Heron Egretta tricolor S 18 Cattle Egret Bubulcus ibis S 19 Green Heron Butorides virescens S 20 Striated Heron Butorides striata S 21 Black-crowned Night-Heron Nycticorax nycticorax S 22 Yellow-crowned Night-Heron Nyctanassa violacea S 23 Boat-billed Heron Cochlearius cochlearius S IBISES AND SPOONBILLS 24 Scarlet Ibis Eudocimus ruber S NEW WORLD VULTURES Page 1 of 8 www.eagle-eye.com Trinidad Tobago Leader: Ernesto Carman Eagle-Eye Tours Nov 29 - Dec 9, 2018 Bird Species Seen/ Common Name Scientific Name Heard 25 Black Vulture Coragyps atratus S 26 Turkey Vulture Cathartes aura S OSPREY 27 Osprey Pandion haliaetus S HAWKS, -
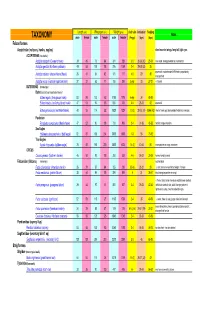
Avian Taxonomy
Length (cm) Wing span (cm) Weight (gms) cluch size incubation fledging Notes TAXONOMY male female male female male female (# eggs) (days) (days) Falconiformes Accipitridae (vultures, hawks, eagles) short rounded wings; long tail; light eyes ACCIPITRINAE (true hawks) Accipiter cooperii (Cooper's hawk) 39 45 73 84 341 528 3-5 30-36 (30) 25-34 crow sized; strongly banded tail; rounded tail Accipiter gentilis (Northern goshawk) 49 58 101 108 816 1059 2-4 28-38 (33) 35 square tail; most abundant NAM hawk; proportionaly 26 31 54 62 101 177 4-5 29 30 Accipiter striatus (sharp-shinned hawk) strongest foot Accipiter nisus (Eurasian sparrowhawk ) 37 37 62 77 150 290 5-Apr 33 27-31 Musket BUTEONINAE (broadwings) Buteo (buzzards or broad tailed hawks) Buteo regalis (ferruginous hawk) 53 59 132 143 1180 1578 6-Apr 34 45-50 Buteo lineatus (red-shouldered hawk) 47 53 96 105 550 700 3-4 28-33 42 square tail Buteo jamaicensis (red-tailed hawk) 48 55 114 122 1028 1224 1-3 (3) 28-35 (34) 42-46 (42) (Harlan' hawk spp); dark patagial featehres: immature; Parabuteo Parabuteo cuncincutus (Harris hawk) 47 52 90 108 710 890 2-4 31-36 45-50 reddish orange shoulders Sea Eagles Haliaeetus leucocephalus (bald eagle) 82 87 185 244 3000 6300 1-3 35 70-92 True Eagles Aquila chrysaetos (golden eagle) 78 82 185 220 3000 6125 1-4 (2) 40-45 50 white patches on wings: immature; CIRCUS Circus cyaneus (Northern harrier) 46 50 93 108 350 530 4-6 26-32 30-35 hovers; hunts by sound Falconidae (falcons) (longwings) notched beak Falco columbarius (American merlin) 26 29 57 64 -

Estimations Relative to Birds of Prey in Captivity in the United States of America
ESTIMATIONS RELATIVE TO BIRDS OF PREY IN CAPTIVITY IN THE UNITED STATES OF AMERICA by Roger Thacker Department of Animal Laboratories The Ohio State University Columbus, Ohio 43210 Introduction. Counts relating to birds of prey in captivity have been accomplished in some European countries; how- ever, to the knowledge of this author no such information is available in the United States of America. The following paper consistsof data related to this subject collected during 1969-1970 from surveys carried out in many different direc- tions within this country. Methods. In an attempt to obtain as clear a picture as pos- sible, counts were divided into specific areas: Research, Zoo- logical, Falconry, and Pet Holders. It became obvious as the project advanced that in some casesthere was overlap from one area to another; an example of this being a falconer working with a bird both for falconry and research purposes. In some instances such as this, the author has used his own judgment in placing birds in specific categories; in other in- stances received information has been used for this purpose. It has also become clear during this project that a count of "pets" is very difficult to obtain. Lack of interest, non-coop- eration, or no available information from animal sales firms makes the task very difficult, as unfortunately, to obtain a clear dispersal picture it is from such sourcesthat informa- tion must be gleaned. However, data related to the importa- tion of birds' of prey as recorded by the Bureau of Sport Fisheries and Wildlife is included, and it is felt some observa- tions can be made from these figures. -

Harris Hawk Parabuteo Unicinctus
Harris Hawk Parabuteo unicinctus Class: Aves Order: Falconiformes Family: Accipitridae Characteristics: As their Spanish name aguililla rojinegra (little red & black bird of prey) alludes to, the Harris Hawk’s flight feathers, tail feathers, dorsal and undercarriage are composed of a very dark brown color while their wings and legs are covered in a rusty red color. Unlike the Spanish name suggests, the Harris Hawk is considered a hawk. Their Latin name refers to their vulture-like wide, thick wings and the belt of white plumage at the base and the end of their tails. Behavior: Harris Hawks are the most social of the North American raptors, forming non-migratory, territorial groups composed of 2 to 7 (most commonly 4) individuals (All About Birds). With larger groups, there is usually an alpha female that is dominant over all the other group members including the alpha male. There is a small possibility of a subordinate breeding alpha female (Bednarz 1986, Dawson and Mannan 1991). Harris Hawk groups often hunt together. During the hunt, it has been observed that the hawks will communicate with one another via twitching the white markings on the tail (Woburn Safari Park). Hunting affords them a decrease in energy expenditure, the ability to successfully scare out and capture prey from hiding places and the ability to hunt larger prey than themselves (Bednarz 1988, Coulson and Coulson 2013). Reproduction: The alpha female and male are most often the only breeders of the group. Females frequently have clutches during the late Spring/early summer months, but can have up to three clutches per year. -

Timing and Abundance of Grey-Faced Buzzards Butastur Indicus and Other Raptors on Northbound Migration in Southern Thailand, Spring 2007–2008
FORKTAIL 25 (2009): 90–95 Timing and abundance of Grey-faced Buzzards Butastur indicus and other raptors on northbound migration in southern Thailand, spring 2007–2008 ROBERT DeCANDIDO and CHUKIAT NUALSRI We provide the first extensive migration data about northbound migrant raptors in Indochina. Daily counts were made at one site (Promsri Hill) in southern Thailand near the city of Chumphon, from late February through early April 2007–08. We identified 19 raptor species as migrants, and counted 43,451 individuals in 2007 (112.0 migrants/hr) and 55,088 in 2008 (160.6 migrants/hr), the highest number of species and seasonal totals for any spring raptor watch site in the region. In both years, large numbers of raptors were first seen beginning at 12h00, and more than 70% of the migration was observed between 14h00 and 17h00 with the onset of strong thermals and an onshore sea breeze from the nearby Gulf of Thailand. Two raptor species, Jerdon’s Baza Aviceda jerdoni and Crested Serpent Eagle Spilornis cheela, were recorded as northbound migrants for the first time in Asia. Four species composed more than 95% of the migration: Black Baza Aviceda leuphotes (mean 50.8 migrants/hr in 2007–08), Grey-faced Buzzard Butastur indicus (47.5/hr in 2007–08), Chinese Sparrowhawk Accipiter soloensis (22.3/hr in 2007–08), and Oriental Honey-buzzard Pernis ptilorhynchus (7.5/hr in 2007–08). Most (>95%) Black Bazas, Chinese Sparrowhawks and Grey-faced Buzzards were observed migrating in flocks. Grey-faced Buzzard flocks averaged 25–30 birds/ flock. Seasonally, our counts indicate that the peak of the Grey-faced Buzzard migration occurs in early to mid-March, while Black Baza and Chinese Sparrowhawk peak in late March through early April.