PDF (Chapter 11. Evolution of the Mantle)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mantle Transition Zone Structure Beneath Northeast Asia from 2-D
RESEARCH ARTICLE Mantle Transition Zone Structure Beneath Northeast 10.1029/2018JB016642 Asia From 2‐D Triplicated Waveform Modeling: Key Points: • The 2‐D triplicated waveform Implication for a Segmented Stagnant Slab fi ‐ modeling reveals ne scale velocity Yujing Lai1,2 , Ling Chen1,2,3 , Tao Wang4 , and Zhongwen Zhan5 structure of the Pacific stagnant slab • High V /V ratios imply a hydrous p s 1State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, and/or carbonated MTZ beneath 2 3 Northeast Asia Beijing, China, College of Earth Sciences, University of Chinese Academy of Sciences, Beijing, China, CAS Center for • A low‐velocity gap is detected within Excellence in Tibetan Plateau Earth Sciences, Beijing, China, 4Institute of Geophysics and Geodynamics, School of Earth the stagnant slab, probably Sciences and Engineering, Nanjing University, Nanjing, China, 5Seismological Laboratory, California Institute of suggesting a deep origin of the Technology, Pasadena, California, USA Changbaishan intraplate volcanism Supporting Information: Abstract The structure of the mantle transition zone (MTZ) in subduction zones is essential for • Supporting Information S1 understanding subduction dynamics in the deep mantle and its surface responses. We constructed the P (Vp) and SH velocity (Vs) structure images of the MTZ beneath Northeast Asia based on two‐dimensional ‐ Correspondence to: (2 D) triplicated waveform modeling. In the upper MTZ, a normal Vp but 2.5% low Vs layer compared with L. Chen and T. Wang, IASP91 are required by the triplication data. In the lower MTZ, our results show a relatively higher‐velocity [email protected]; layer (+2% V and −0.5% V compared to IASP91) with a thickness of ~140 km and length of ~1,200 km [email protected] p s atop the 660‐km discontinuity. -

Deep Earth Structure: Lower Mantle and D"
This article was originally published in Treatise on Geophysics, Second Edition, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial Lay T Deep Earth Structure: Lower Mantle and D″. In: Gerald Schubert (editor-in-chief) Treatise on Geophysics, 2nd edition, Vol 1. Oxford: Elsevier; 2015. p. 683-723. Author's personal copy 00 1.22 Deep Earth Structure: Lower Mantle and D T Lay, University of California Santa Cruz, Santa Cruz, CA, USA ã 2015 Elsevier B.V. All rights reserved. 1.22.1 Lower Mantle and D00 Basic Structural Attributes 684 1.22.1.1 Elastic Parameters, Density, and Thermal Structure 684 1.22.1.2 Mineralogical Structure 685 1.22.2 One-Dimensional Lower Mantle Structure 686 1.22.2.1 Body-Wave Travel Time and Slowness Constraints 687 1.22.2.2 Surface-Wave/Normal-Mode Constraints 688 1.22.2.3 Attenuation Structure 688 1.22.3 Three-Dimensional -

Chemical Composition of Earth's Primitive Mantle and Its Variance: 2
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112, B03212, doi:10.1029/2005JB004224, 2007 Click Here for Full Article Chemical composition of Earth’s primitive mantle and its variance: 2. Implications for global geodynamics Tanya Lyubetskaya1 and Jun Korenaga1 Received 16 December 2005; revised 17 June 2006; accepted 20 November 2006; published 29 March 2007. [1] The global budgets of argon and heat-producing elements have traditionally been used to argue for layered-mantle convection because they require a large fraction of Earth’s mantle to remain isolated from mantle convection. We revise these mass balance arguments using our new composition model of the primitive mantle and show that the global budgets of argon, heat-producing elements, and rare earth elements are consistent with Earth’s mantle almost entirely composed of the mid-ocean ridge basalt source mantle, supporting the notion of whole mantle convection. Combined with a recent theory on the thermal evolution of Earth, our revised thermal budget implies inefficient mixing and processing in the past, explaining the survival of long-lived geochemical heterogeneities in convecting mantle. Citation: Lyubetskaya, T., and J. Korenaga (2007), Chemical composition of Earth’s primitive mantle and its variance: 2. Implications for global geodynamics, J. Geophys. Res., 112, B03212, doi:10.1029/2005JB004224. 1. Introduction arguments do not provide a robust constraint on the size of such a hidden reservoir, because the interpretation of [2] The structure of mantle convection has been one of geochemical data is often model-dependent. For example, the most controversial subjects in modern geodynamics and observed geochemical differences between mantle-derived geochemistry. -

Geochemical Behaviour of Trace Elements During Fractional
Anais da Academia Brasileira de Ciências (2015) 87(4): 1959-1979 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201520130385 www.scielo.br/aabc Geochemical behaviour of trace elements during fractional crystallization and crustal assimilation of the felsic alkaline magmas of the state of Rio de Janeiro, Brazil AKIHISA Motoki1*, SUSANNA E. SICHEL2, THAIS Vargas1, DEAN P. MELO3 and KENJI F. Motoki2 1Departamento de Mineralogia e Petrologia Ígnea, Universidade do Estado do Rio de Janeiro, Rua São Francisco Xavier, 524, Sala A-4023, Maracanã, 20550-990 Rio de Janeiro, RJ, Brasil 2Departamento de Geologia, Universidade Federal Fluminense, Av. General Milton Cardoso, s/n, 4° Andar, Gragoatá, 24210-340 Niterói, RJ, Brasil 3PETROBRAS, CENPES, Av. Horácio Macedo, 950, Cidade Universitária, 21941-915 Rio de Janeiro, RJ, Brasil Manuscript received on September 24, 2013; accepted for publication on June 10, 2015 ABSTRACT This paper presents geochemical behaviour of trace elements of the felsic alkaline rocks of the state of Rio de Janeiro, Brazil, with special attention of fractional crystallization and continental crust assimilation. Fractionation of leucite and K-feldspar increases Rb/K and decreases K2O/(K2O+Na2O). Primitive nepheline syenite magmas have low Zr/TiO2, Sr, and Ba. On the Nb/Y vs. Zr/TiO2 diagram, these rocks are projected on the field of alkaline basalt, basanite, and nephelinite, instead of phonolite. Well-fractionated peralkaline nepheline syenite has high Zr/TiO2 but there are no zircon. The diagrams of silica saturation index (SSI) distinguish the trends originated form fractional crystallization and crustal assimilation. -

Chapter 10: Mantle Melting and the Generation of Basaltic Magma 2 Principal Types of Basalt in the Ocean Basins Tholeiitic Basalt and Alkaline Basalt
Chapter 10: Mantle Melting and the Generation of Basaltic Magma 2 principal types of basalt in the ocean basins Tholeiitic Basalt and Alkaline Basalt Table 10.1 Common petrographic differences between tholeiitic and alkaline basalts Tholeiitic Basalt Alkaline Basalt Usually fine-grained, intergranular Usually fairly coarse, intergranular to ophitic Groundmass No olivine Olivine common Clinopyroxene = augite (plus possibly pigeonite) Titaniferous augite (reddish) Orthopyroxene (hypersthene) common, may rim ol. Orthopyroxene absent No alkali feldspar Interstitial alkali feldspar or feldspathoid may occur Interstitial glass and/or quartz common Interstitial glass rare, and quartz absent Olivine rare, unzoned, and may be partially resorbed Olivine common and zoned Phenocrysts or show reaction rims of orthopyroxene Orthopyroxene uncommon Orthopyroxene absent Early plagioclase common Plagioclase less common, and later in sequence Clinopyroxene is pale brown augite Clinopyroxene is titaniferous augite, reddish rims after Hughes (1982) and McBirney (1993). Each is chemically distinct Evolve via FX as separate series along different paths Tholeiites are generated at mid-ocean ridges Also generated at oceanic islands, subduction zones Alkaline basalts generated at ocean islands Also at subduction zones Sources of mantle material Ophiolites Slabs of oceanic crust and upper mantle Thrust at subduction zones onto edge of continent Dredge samples from oceanic crust Nodules and xenoliths in some basalts Kimberlite xenoliths Diamond-bearing pipes blasted up from the mantle carrying numerous xenoliths from depth Lherzolite is probably fertile unaltered mantle Dunite and harzburgite are refractory residuum after basalt has been extracted by partial melting 15 Tholeiitic basalt 10 5 Figure 10-1 Brown and Mussett, A. E. (1993), The Inaccessible Earth: An Integrated View of Its Lherzolite Structure and Composition. -

Partial Melting - Simple Process, Huge Global Impact How Partial Melting, Coupled with Plate Tectonics, Has Changed the Chemistry of Our Planet
Earthlearningidea - http://www.earthlearningidea.com/ Partial melting - simple process, huge global impact How partial melting, coupled with plate tectonics, has changed the chemistry of our planet Demonstrating partial melting Explaining the planetary effects of partial melting Prepare two small beakers as described in the Each time that partial melting takes place during different resources section below. stages of the plate tectonic cycle, materials with different chemical and physical makeup are formed. Pre- prepared The starting point for these processes is the mantle, beakers where the most abundant elements are oxygen, silicon, magnesium and iron in that order. However the Earth’s crust contains much more silicon and oxygen and much less magnesium and iron than the mantle, and is formed through these stages: Photo: C. King. Stage 1: the mantle partially melts beneath oceanic ridges; the melt formed is richer in oxygen/silicon (and poorer in iron/magnesium) than the original mantle rock. This rises and then cools to form new oceanic crust and Show the pupils the beaker containing the mixture of ocean ridge volcanoes, as plates move apart. gravel and chopped candlewax before heating; ask what will happen if the beaker is heated until the wax Stage 2: the oceanic crust subducted beneath melts. Most will realise that the gravel will sink to the oceanic plates partially melts; the melt is still richer in bottom to form a mixed gravel/wax layer, whilst pure oxygen/silicon (and poorer in iron/ magnesium) than the wax will flow to the top to form another layer. Then original oceanic crust rock. -

Scientific Research of the Sco Countries: Synergy and Integration 上合组织国家的科学研究:协同和一体化
SCIENTIFIC RESEARCH OF THE SCO COUNTRIES: SYNERGY AND INTEGRATION 上合组织国家的科学研究:协同和一体化 Materials of the Date: International Conference November 19 Beijing, China 2019 上合组织国家的科学研究:协同和一体化 国际会议 参与者的英文报告 International Conference “Scientific research of the SCO countries: synergy and integration” Part 1: Participants’ reports in English 2019年11月19日。中国北京 November 19, 2019. Beijing, PRC Materials of the International Conference “Scientific research of the SCO countries: synergy and integration”. Part 1 - Reports in English (November 19, 2019. Beijing, PRC) ISBN 978-5-905695-74-2 这些会议文集结合了会议的材料 - 研究论文和科学工作 者的论文报告。 它考察了职业化人格的技术和社会学问题。 一些文章涉及人格职业化研究问题的理论和方法论方法和原 则。 作者对所引用的出版物,事实,数字,引用,统计数据,专 有名称和其他信息的准确性负责 These Conference Proceedings combine materials of the conference – research papers and thesis reports of scientific workers. They examines tecnical and sociological issues of research issues. Some articles deal with theoretical and methodological approaches and principles of research questions of personality professionalization. Authors are responsible for the accuracy of cited publications, facts, figures, quotations, statistics, proper names and other information. ISBN 978-5-905695-74-2 © Scientific publishing house Infinity, 2019 © Group of authors, 2019 CONTENTS ECONOMICS 可再生能源发展的现状与前景 Current state and prospects of renewable energy sources development Linnik Vladimir Yurievich, Linnik Yuri Nikolaevich...........................................12 JURISPRUDENCE 法律领域的未命名合同 Unnamed contracts in the legal field Askarov Nosirjon Ibragimovich...........................................................................19 -

Mantle Plumes
Geol. 655 Isotope Geochemistry Lecture 21 Spring 2007 ISOTOPIC EVOLUTION OF THE MANTLE IV THE ORIGIN OF MANTLE PLUMES AND THE COMMON COMPONENT IN PLUMES Determining how the various geochemical reservoirs of the mantle have evolved is among the most vexing problems in geochemistry. The principal observation to be explained is that mantle plumes in- variably have less depleted isotopic signatures than MORB, and the isotopic compositions of some in- dicate net enrichment in incompatible elements. As we saw in the previous lecture, mantle plumes were initially thought to consist of primitive mantle (e.g., Schilling, 1973). As we found, mixing be- tween primitive and depleted mantle can explain the Sr and Nd isotopic compositions of some plumes, but virtually none of the Pb isotope data can be explained this way, nor are the trace element composi- tions of OIB consistent with plumes being composed of primitive mantle. Indeed, although ‘primitive mantle’ has proved to be a useful hypothetical concept, no mantle-derived basalts or xenoliths have appropriate compositions to be ‘primitive mantle’ or derived from it. It is possible that no part of the mantle retains its original, primitive, composition (on the other hand, to have survived, primitive man- tle must not participate in volcanism and other such processes, so the absence of evidence for a primi- tive mantle reservoir is not evidence of its absence). Hofmann and White (1982) suggested mantle plumes obtain their unique geochemical signature through deep recycling of oceanic crust (Figure 21.1). Partial melting at mid-ocean ridges creates oce- anic crust that is less depleted in incompatible elements than the depleted upper mantle. -

Experimental Partitioning of Rb, Cs, Sr, and Ba Between Alkali Feldspar and Peraluminous Melt
American Mineralogist, Volume 81, pages 719-734,1996 Experimental partitioning of Rb, Cs, Sr, and Ba between alkali feldspar and peraluminous melt JONATHAN IcENHOWER AND DAVID LoNDON School of Geology and Geophysics, University of Oklahoma, 100 East Boyd Street, SEC 810, Norman, Oklahoma 73019, U.S.A. ABSTRACT Hydrous partial melting experiments performed between 650 and 750 °C at 200 MPa (H20) on synthetic metapelite compositions (quartz + albite + muscovite + biotite min- eral mixtures) doped with Ba, Sr, Rb, and Cs yielded alkali feldspar crystals with a wide range of compositions in equilibrium at their rims with peraluminous melt. Measured partition coefficients for normally trace lithophile elements between feldspar and melt [D(M)FSP/gl,M = Ba, Sr, Rb, Cs] do not depend on either temperature or bulk composition of melt for the compositions studied. Values of D(Sr)FSP/glare between 10 and 14 and appear to be independent of the albite and orthoclase contents of the feldspar crystals. In contrast, values of D(Ba)FSP/gland D(Rb)FsP/glare strongly dependent on the orthoclase content of feldspar, relationships that can be expressed by the following linear equations: D(Ba)FSP/gl= 0.07 + 0.25(orthoclase) and D(Rb)FsP/gl= 0.03 + 0.0 1(orthoclase), where orthoclase is in mole percent. These equations reproduce the range of previously reported values for D(Ba) and D(Rb) determined on natural and synthetic samples. A single par- tition coefficient for Cs was also determined at D(CS)Fsp/gl= 0.13. These data can be used in conjunction with recently published partition coefficients for muscovite, biotite, and plagioclase feldspars (Blundy and Wood 1991; Icenhower and London 1995) to model quantitatively the trace element signatures of peraluminous mag- mas during anatexis and crystallization. -

Petrogenesis of Slab-Derived Trondhjemite-Tonalite-Dacite/ Adakite Magmas M
Transactions of the Royal Society of Edinburgh: Earth Sciences, 87, 205-215, 1996 Petrogenesis of slab-derived trondhjemite-tonalite-dacite/ adakite magmas M. S. Drummond, M. J. Defant and P. K. Kepezhinskas ABSTRACT: The prospect of partial melting of the subducted oceanic crust to produce arc magmatism has been debated for over 30 years. Debate has centred on the physical conditions of slab melting and the lack of a definitive, unambiguous geochemical signature and petrogenetic process. Experimental partial melting data for basalt over a wide range of pressures (1-32 kbar) and temperatures (700-1150=C) have shown that melt compositions are primarily trondhjemite- tonalite-dacite (TTD). High-Al (> 15% A12O3 at the 70% SiO2 level) TTD melts are produced by high-pressure 015 kbar) partial melting of basalt, leaving a restite assemblage of garnet + clinopyroxe'ne ± hornblende. A specific Cenozoic high-Al TTD (adakite) contains lower Y, Yb and Sc and higher Sr, Sr/Y, La'/Yb and.Zr/Sm relative to other TTD types and is interpreted to represent a slab melt under garnet amphibolite to eclogite conditions. High-Al TTD with an adakite-like geochemical character is prevalent in the Archean as the result of a higher geotherm that facilitated slab melting. Cenozoic adakite localities are commonly associated with the subduction of young (<25Ma), hot oceanic crust, which may provide a slab geotherm (*9-10=C km"1) conducive for slab dehydration melting. Viable alternative or supporting tectonic effects that may enhance slab melting include highly oblique convergence and resultant high shear stresses and incipient subduction into a pristine hot mantle wedge. -
Using Rare Earth Elements to Model Silicate Melting and Crystallization
Using Rare Earth Elements to Model Silicate Melting and Crystallization The Rare Earth Elements (REE) The lanthanide series is formed by filling of 4f orbitals, and its constituents are commonly termed the rare earth elements (REE). The common valence state is +3 for all REE over a wide range of oxygen fugacity; Ce+4 can occur in highly oxidized environments at the earth's surface, and Eu+2 in reducing environments in the crust and mantle. The rare earth elements are lithophile elements (low electronegativities lead to the formation of highly ionic bonds), which substitute into many silicates and phosphates. Ionic radii of the lanthanides decreases with increasing atomic number from La to Lu, the lanthanide contraction… Because of their high charge and large radii, the rare earth elements are usually relatively incompatible in silicate minerals. However, due to the lanthanide contraction, the heavier rare earths are smaller and thus can "fit" within some lattice sites (although there must be charge compensation for the 3+ valence), so incompatibility decreases with increasing Z. Class Discussion #1: What mineral lattice sites could host the heavy rare earth elements? What about Eu2+? REE diagrams If we plot the concentrations of the rare earth elements as a group on a diagram of abundance versus atomic number, we observe two cosmochemical phenomena: 1) the decrease in absolute abundance with increasing atomic number; 2) the “even-odd effect” in relative abundances (higher abundances of even atomic number elements). Because these cosmochemical effects are common to all terrestrial rocks, we would rather ignore them in order to accentuate more subtle variations in absolute and relative concentrations of REE between samples. -
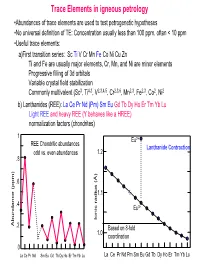
Trace Elements in Igneous Petrology
Trace Elements in igneous petrology •Abundances of trace elements are used to test petrogenetic hypotheses •No universal definition of TE: Concentration usually less than 100 ppm, often < 10 ppm •Useful trace elements: a)First transition series: Sc Ti V Cr Mn Fe Co Ni Cu Zn Ti and Fe are usually major elements, Cr, Mn, and Ni are minor elements Progressive filling of 3d orbitals Variable crystal field stabilization Commonly multivalent (Sc3, Ti4,3, V2,3,4,5, Cr2,3,6, Mn2,3, Fe2,3, Co2, Ni2 b) Lanthanides (REE): La Ce Pr Nd (Pm) Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Light REE and heavy REE (Y behaves like a HREE) normalization factors (chondrites) 1 Eu2+ REE Chondritic abundances Lanthanide Contraction odd vs. even abundances 1.2 .8 .6 1.1 .4 Eu3+ Ionic radius (Å) Ionic radius Abundance (ppm) .2 Based on 8-fold 1.0 coordination 0 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu (c) Large Ion Lithophile Elements (LILE): may also be partitioned into fluid phase Alkalis: K Rb Cs (monovalent) Alkaline earths: Ba Sr (divalent) Actinides: U, Th, Ra, Pa (multiple valency) (d) High field strength elements (HFSE): small, highly-charged ions Zr, Hf (4 valent) Nb, Ta (4 and 5 valent) (e) Chalcophile elements: Cu, Zn, Pb, Ag, Hg, PGE, (Fe, Co, Ni) (f) Siderophile elements: Fe, Ni, Co, Ge, P, Ga, Au (PGE)… • Decoupled from major elements: lack of stoichiometric constraints (not strictly true) • Goldschmidt’s Rules • Generalities: Incompatible elements are elements that tend to be excluded from common minerals (olivines, pyroxenes, garnets, feldspars, oxides…) in equilibrium with a melt, i.e., they have low D values.