Analysis of Gene Expression from Systemic Lupus Erythematosus Synovium Reveals Myeloid Cell‑Driven Pathogenesis of Lupus Arthritis Erika L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mapping Autosomal Recessive Intellectual Disability: Combined Microarray and Exome
bioRxiv preprint doi: https://doi.org/10.1101/092346; this version posted March 15, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Mapping Autosomal Recessive Intellectual Disability: Combined Microarray and Exome Sequencing Identifies 26 Novel Candidate Genes in 192 Consanguineous Families Ricardo Harripaul, MSc1,2, Nasim Vasli, PhD1, Anna Mikhailov, BSc1, Muhammad Arshad Rafiq, PhD 1,3, Kirti Mittal, PhD 1, Christian Windpassinger, PhD4, Taimoor I. Sheikh, MPhil1,2, Abdul Noor, PhD5,6, Huda Mahmood, BSc1, Samantha Downey1,7, Maneesha Johnson1,7, Kayla Vleuten1,7, Lauren Bell1,7, Muhammad Ilyas, M.Phil8, Falak Sher Khan, MS9, Valeed Khan, MS9, Mohammad Moradi, MSc10, Muhammad Ayaz11, Farooq Naeem, PhD 11,12, Abolfazl Heidari, PhD1,13, Iltaf Ahmed, PhD14, Shirin Ghadami, PhD15, Zehra Agha, PhD3, Sirous Zeinali, PhD15, Raheel Qamar, PhD3,16, Hossein Mozhdehipanah, MD17, Peter John, PhD14, Asif Mir, PhD8, Muhammad Ansar, PhD9, Leon French, PhD18, Muhammad Ayub, MBBS, MD11,12, John B. Vincent, PhD1,2,19 1Molecular Neuropsychiatry & Development (MiND) Lab, Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada; 2Institute of Medical Science, University of Toronto, Toronto, ON, Canada; 3Dept. of Biosciences, COMSATS Institute of Information Technology, Islamabad, Pakistan; 4Institute of Human Genetics, Medical University of Graz, Graz, Austria; 5Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto, ON, Canada; 6 Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON, Canada; 1 bioRxiv preprint doi: https://doi.org/10.1101/092346; this version posted March 15, 2017. -

Genetic Variations in the PSMA6 and PSMC6 Proteasome Genes Are Associated with Multiple Sclerosis and Response to Interferon‑Β Therapy in Latvians
EXPERIMENTAL AND THERAPEUTIC MEDICINE 21: 478, 2021 Genetic variations in the PSMA6 and PSMC6 proteasome genes are associated with multiple sclerosis and response to interferon‑β therapy in Latvians NATALIA PARAMONOVA1, JOLANTA KALNINA1, KRISTINE DOKANE1, KRISTINE DISLERE1, ILVA TRAPINA1, TATJANA SJAKSTE1 and NIKOLAJS SJAKSTE1,2 1Genomics and Bioinformatics, Institute of Biology of The University of Latvia; 2Department of Medical Biochemistry of The University of Latvia, LV‑1004 Riga, Latvia Received July 8, 2020; Accepted December 8, 2020 DOI: 10.3892/etm.2021.9909 Abstract. Several polymorphisms in genes related to the Introduction ubiquitin‑proteasome system exhibit an association with pathogenesis and prognosis of various human autoimmune Multiple sclerosis (MS) is a lifelong demyelinating disease of diseases. Our previous study reported the association the central nervous system. The clinical onset of MS tends to between multiple sclerosis (MS) and the PSMA3‑rs2348071 be between the second and fourth decade of life. Similarly to polymorphism in the Latvian population. The current study other autoimmune diseases, women are affected 3‑4 times more aimed to evaluate the PSMA6 and PSMC6 genetic variations, frequently than men (1). About 10% of MS patients experience their interaction between each other and with the rs2348071, a primary progressive MS form characterized by the progres‑ on the susceptibility to MS risk and response to therapy in sion of neurological disability from the onset. In about 90% the Latvian population. PSMA6‑rs2277460, ‑rs1048990 and of MS patients, the disease undergoes the relapse‑remitting PSMC6‑rs2295826, ‑rs2295827 were genotyped in the MS MS course (RRMS); in most of these patients, the condition case/control study and analysed in terms of genotype‑protein acquires secondary progressive course (SPMS) (2). -

Differential Expression of Interferon-Γ and Chemokine Genes
Differential expression of interferon-γ and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response Owens et al. Owens et al. Journal of Neuroinflammation 2013, 10:56 http://www.jneuroinflammation.com/content/10/1/56 Owens et al. Journal of Neuroinflammation 2013, 10:56 JOURNAL OF http://www.jneuroinflammation.com/content/10/1/56 NEUROINFLAMMATION RESEARCH Open Access Differential expression of interferon-γ and chemokine genes distinguishes Rasmussen encephalitis from cortical dysplasia and provides evidence for an early Th1 immune response Geoffrey C Owens1,7*, My N Huynh1, Julia W Chang1, David L McArthur1, Michelle J Hickey2, Harry V Vinters2,3, Gary W Mathern1,4,5,6† and Carol A Kruse1,6† Abstract Background: Rasmussen encephalitis (RE) is a rare complex inflammatory disease, primarily seen in young children, that is characterized by severe partial seizures and brain atrophy. Surgery is currently the only effective treatment option. To identify genes specifically associated with the immunopathology in RE, RNA transcripts of genes involved in inflammation and autoimmunity were measured in brain tissue from RE surgeries and compared with those in surgical specimens of cortical dysplasia (CD), a major cause of intractable pediatric epilepsy. Methods: Quantitative polymerase chain reactions measured the relative expression of 84 genes related to inflammation and autoimmunity in 12 RE specimens and in the reference group of 12 CD surgical specimens. Data were analyzed by consensus clustering using the entire dataset, and by pairwise comparison of gene expression levels between the RE and CD cohorts using the Harrell-Davis distribution-free quantile estimator method. -

Supplementary Information
Supplementary Information This text file includes: Supplementary Methods Supplementary Figure 1-13, 15-30 Supplementary Table 1-8, 16, 20-21, 23, 25-37, 40-41 1 1. Samples, DNA extraction and genome sequencing 1.1 Ethical statements and sample storage The ethical statements of collecting and processing tissue samples for each species are listed as follows: Myotis myotis: All procedures were carried out in accordance with the ethical guidelines and permits (AREC-13-38-Teeling) delivered by the University College Dublin and the Préfet du Morbihan, awarded to Emma Teeling and Sébastien Puechmaille respectively. A single M. myotis individual was humanely sacrificed given that she had lethal injuries, and dissected. Rhinolophus ferrumequinum: All the procedures were conducted under the license (Natural England 2016-25216-SCI-SCI) issued to Gareth Jones. The individual bat died unexpectedly and suddenly during sampling and was dissected immediately. Pipistrellus kuhlii: The sampling procedure was carried out following all the applicable national guidelines for the care and use of animals. Sampling was done in accordance with all the relevant wildlife legislation and approved by the Ministry of Environment (Ministero della Tutela del Territorio e del Mare, Aut.Prot. N˚: 13040, 26/03/2014). Molossus molossus: All sampling methods were approved by the Ministerio de Ambiente de Panamá (SE/A-29-18) and by the Institutional Animal Care and Use Committee of the Smithsonian Tropical Research Institute (2017-0815-2020). Phyllostomus discolor: P. discolor bats originated from a breeding colony in the Department Biology II of the Ludwig-Maximilians-University in Munich. Approval to keep and breed the bats was issued by the Munich district veterinary office. -
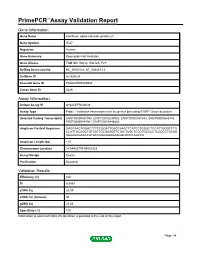
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name interferon, alpha-inducible protein 27 Gene Symbol IFI27 Organism Human Gene Summary Description Not Available Gene Aliases FAM14D, ISG12, ISG12A, P27 RefSeq Accession No. NC_000014.8, NT_026437.12 UniGene ID Hs.532634 Ensembl Gene ID ENSG00000165949 Entrez Gene ID 3429 Assay Information Unique Assay ID qHsaCEP0024638 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000557098, ENST00000298902, ENST00000557634, ENST00000555744, ENST00000444961, ENST00000448882 Amplicon Context Sequence GAGCAACTGGACTCTCCGGATTGACCAAGTTCATCCTGGGCTCCATTGGGTCTG CCATTGCGGCTGTCATTGCGAGGTTCTACTAGCTCCCTGCCCCTCGCCCTGCAG AGAAGAGAACCATGCCAGGGGAGAAGGCACCCAGCCA Amplicon Length (bp) 115 Chromosome Location 14:94582779-94582923 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 100 R2 0.9997 cDNA Cq 23.09 cDNA Tm (Celsius) 86 gDNA Cq 23.06 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report IFI27, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Human Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. Detected Coding Transcript(s) This is a list of the Ensembl transcript ID(s) that this assay will detect. -

Cell Adhesion and Specificity
RESEARCH ARTICLE Molecular basis of sidekick-mediated cell- cell adhesion and specificity Kerry M Goodman1†, Masahito Yamagata2,3†, Xiangshu Jin1,4‡, Seetha Mannepalli1, Phinikoula S Katsamba4,5, Go¨ ran Ahlse´ n4,5, Alina P Sergeeva4,5, Barry Honig1,4,5,6,7*, Joshua R Sanes2,3*, Lawrence Shapiro1,5,7* 1Department of Biochemistry and Molecular Biophysics, Columbia University, New York, United States; 2Department of Molecular and Cellular Biology, Harvard University, Cambridge, United States; 3Center for Brain Science, Harvard University, Cambridge, United States; 4Howard Hughes Medical Institute, Columbia University, New York, United States; 5Department of Systems Biology, Columbia University, New York, United States; 6Department of Medicine, Columbia University, New York, United States; 7Zuckerman Mind Brain and Behavior Institute, Columbia University, New York, United States *For correspondence: bh6@cumc. Abstract Sidekick (Sdk) 1 and 2 are related immunoglobulin superfamily cell adhesion proteins columbia.edu (BH); sanesj@mcb. required for appropriate synaptic connections between specific subtypes of retinal neurons. Sdks harvard.edu (JRS); shapiro@ mediate cell-cell adhesion with homophilic specificity that underlies their neuronal targeting convex.hhmi.columbia.edu (LS) function. Here we report crystal structures of Sdk1 and Sdk2 ectodomain regions, revealing similar †These authors contributed homodimers mediated by the four N-terminal immunoglobulin domains (Ig1–4), arranged in a equally to this work horseshoe conformation. These Ig1–4 horseshoes interact in a novel back-to-back orientation in both homodimers through Ig1:Ig2, Ig1:Ig1 and Ig3:Ig4 interactions. Structure-guided mutagenesis Present address: ‡Department results show that this canonical dimer is required for both Sdk-mediated cell aggregation (via trans of Chemistry, Michigan State interactions) and Sdk clustering in isolated cells (via cis interactions). -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Modulating Antiviral Response Via CRISPR–Cas Systems
viruses Review Immunity and Viral Infections: Modulating Antiviral Response via CRISPR–Cas Systems Sergey Brezgin 1,2,3,† , Anastasiya Kostyusheva 1,†, Ekaterina Bayurova 4 , Elena Volchkova 5, Vladimir Gegechkori 6 , Ilya Gordeychuk 4,7, Dieter Glebe 8 , Dmitry Kostyushev 1,3,*,‡ and Vladimir Chulanov 1,3,5,‡ 1 National Medical Research Center of Tuberculosis and Infectious Diseases, Ministry of Health, 127994 Moscow, Russia; [email protected] (S.B.); [email protected] (A.K.); [email protected] (V.C.) 2 Institute of Immunology, Federal Medical Biological Agency, 115522 Moscow, Russia 3 Scientific Center for Genetics and Life Sciences, Division of Biotechnology, Sirius University of Science and Technology, 354340 Sochi, Russia 4 Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products of Russian Academy of Sciences, 108819 Moscow, Russia; [email protected] (E.B.); [email protected] (I.G.) 5 Department of Infectious Diseases, Sechenov University, 119991 Moscow, Russia; [email protected] 6 Department of Pharmaceutical and Toxicological Chemistry, Sechenov University, 119991 Moscow, Russia; [email protected] 7 Department of Organization and Technology of Immunobiological Drugs, Sechenov University, 119991 Moscow, Russia 8 National Reference Center for Hepatitis B Viruses and Hepatitis D Viruses, Institute of Medical Virology, Justus Liebig University of Giessen, 35392 Giessen, Germany; [email protected] * Correspondence: [email protected] † Co-first authors. Citation: Brezgin, S.; Kostyusheva, ‡ Co-senior authors. A.; Bayurova, E.; Volchkova, E.; Gegechkori, V.; Gordeychuk, I.; Glebe, Abstract: Viral infections cause a variety of acute and chronic human diseases, sometimes resulting D.; Kostyushev, D.; Chulanov, V. Immunity and Viral Infections: in small local outbreaks, or in some cases spreading across the globe and leading to global pandemics. -

IFI27 May Predict and Evaluate the Severity of Respiratory Syncytial Virus
Gao et al. Hereditas (2021) 158:3 https://doi.org/10.1186/s41065-020-00167-5 RESEARCH Open Access IFI27 may predict and evaluate the severity of respiratory syncytial virus infection in preterm infants Junyan Gao1, Xueping Zhu2, Mingfu Wu1, Lijun Jiang1, Fudong Wang1 and Shan He2,3* Abstract Background: Preterm infants are a special population that vulnerable to respiratory syncytial virus (RSV) infection and the lower respiratory tract infections (LRTIs) caused by RSV could be severe and even life-threating. The purpose of the present study was to identify candidate genes of preterm infants who are susceptible to RSV infection and provide a new insight into the pathogenesis of RSV infection. Methods: Three datasets (GSE77087, GSE69606 and GSE41374) containing 183 blood samples of RSV infected patients and 33 blood samples of healthy controls from Gene Expression Omnibus (GEO) database were downloaded and the differentially expressed genes (DEGs) were screened out. The function and pathway enrichments were analyzed through Database for Annotation, Visualization and Integrated Discovery (DAVID) website. The protein-protein interaction (PPI) network for DEGs was constructed through Search Tool for the Retrieval of Interacting Genes (STRING). The module analysis was performed by Cytoscape software and hub genes were identified. Clinical verification was employed to verify the expression level of top five hub genes among 72 infants including 50 RSV infected patients and 22 non-RSV-infected patients hospitalized in our center. Further, the RSV infected infants with high-expression IFI27 and those with low-expression IFI27 were compared (defined as higher or lower than the median mRNA level). -

KIAA0001, P2Y Protein-Coupled Rece
Human Immature Monocyte-Derived Dendritic Cells Express the G Protein-Coupled Receptor GPR105 (KIAA0001, P2Y 14) and Increase This information is current as Intracellular Calcium in Response to its of September 29, 2021. Agonist, Uridine Diphosphoglucose Lisa Skelton, Mike Cooper, Marianne Murphy and Adam Platt Downloaded from J Immunol 2003; 171:1941-1949; ; doi: 10.4049/jimmunol.171.4.1941 http://www.jimmunol.org/content/171/4/1941 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2003/08/01/171.4.1941.DC1 Material References This article cites 45 articles, 21 of which you can access for free at: http://www.jimmunol.org/content/171/4/1941.full#ref-list-1 Why The JI? Submit online. by guest on September 29, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2003 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Human Immature Monocyte-Derived Dendritic Cells Express the G Protein-Coupled Receptor GPR105 (KIAA0001, P2Y14) and Increase Intracellular Calcium in Response to its Agonist, Uridine Diphosphoglucose Lisa Skelton,* Mike Cooper,† Marianne Murphy,* and Adam Platt1† Dendritic cells (DC) are essential to the initiation of an immune response due to their unique ability to take-up and process Ag, translocate to lymph nodes, and present processed Ag to naive T cells. -

IFI27 May Predict and Evaluate the Severity of Respiratory Syncytial Virus Infection in Preterm Infants
IFI27 May Predict and Evaluate the Severity of Respiratory Syncytial Virus Infection in Preterm Infants Junyan Gao Aliated Hospital of Yangzhou University https://orcid.org/0000-0001-6678-6147 Xueping Zhu Children's Hospital of Soochow University Mingfu Wu Aliated Hospital of Yangzhou University Lijun Jiang Aliated Hospital of Yangzhou University Fudong Wang Aliated Hospital of Yangzhou University Shan He ( [email protected] ) Research Keywords: respiratory syncytial virus, preterm infants, differentially expressed genes, protein‐protein interaction, pathway enrichment, interferon alpha inducible protein 27 Posted Date: July 15th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-42880/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on January 2nd, 2021. See the published version at https://doi.org/10.1186/s41065-020-00167-5. Page 1/21 Abstract Background: Preterm infants are a special population that vulnerable to respiratory syncytial virus (RSV) infection and the lower respiratory tract infection (LRTIs) caused by RSV could be severe and even life- threating. The purpose of the present study was to identify candidate genes of preterm infants who are susceptible to RSV infection and provide a new insight into the pathogenesis of RSV infection. Methods: Three datasets (GSE77087, GSE69606 and GSE41374) containing 183 blood samples of RSV infected patients and 33 blood samples of healthy controls from Gene Expression Omnibus (GEO) database were downloaded and the differentially expressed genes (DEGs) were screened out. The function and pathway enrichments were analyzed through Database for Annotation, Visualization and Integrated Discovery (DAVID) website. -

Whole Exome Sequencing in Families at High Risk for Hodgkin Lymphoma: Identification of a Predisposing Mutation in the KDR Gene
Hodgkin Lymphoma SUPPLEMENTARY APPENDIX Whole exome sequencing in families at high risk for Hodgkin lymphoma: identification of a predisposing mutation in the KDR gene Melissa Rotunno, 1 Mary L. McMaster, 1 Joseph Boland, 2 Sara Bass, 2 Xijun Zhang, 2 Laurie Burdett, 2 Belynda Hicks, 2 Sarangan Ravichandran, 3 Brian T. Luke, 3 Meredith Yeager, 2 Laura Fontaine, 4 Paula L. Hyland, 1 Alisa M. Goldstein, 1 NCI DCEG Cancer Sequencing Working Group, NCI DCEG Cancer Genomics Research Laboratory, Stephen J. Chanock, 5 Neil E. Caporaso, 1 Margaret A. Tucker, 6 and Lynn R. Goldin 1 1Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 2Cancer Genomics Research Laboratory, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 3Ad - vanced Biomedical Computing Center, Leidos Biomedical Research Inc.; Frederick National Laboratory for Cancer Research, Frederick, MD; 4Westat, Inc., Rockville MD; 5Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; and 6Human Genetics Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA ©2016 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol.2015.135475 Received: August 19, 2015. Accepted: January 7, 2016. Pre-published: June 13, 2016. Correspondence: [email protected] Supplemental Author Information: NCI DCEG Cancer Sequencing Working Group: Mark H. Greene, Allan Hildesheim, Nan Hu, Maria Theresa Landi, Jennifer Loud, Phuong Mai, Lisa Mirabello, Lindsay Morton, Dilys Parry, Anand Pathak, Douglas R. Stewart, Philip R. Taylor, Geoffrey S. Tobias, Xiaohong R. Yang, Guoqin Yu NCI DCEG Cancer Genomics Research Laboratory: Salma Chowdhury, Michael Cullen, Casey Dagnall, Herbert Higson, Amy A.