Competition Between Chalcogen Bond and Halogen Bond Interactions in YOX4:NH3 (Y 5 S, Se; X 5 F, Cl, Br) Complexes: an Ab Initio Investigation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Noncovalent Bonds Through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons
molecules Review Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons Wiktor Zierkiewicz 1,* , Mariusz Michalczyk 1,* and Steve Scheiner 2 1 Faculty of Chemistry, Wrocław University of Science and Technology, Wybrzeze˙ Wyspia´nskiego27, 50-370 Wrocław, Poland 2 Department of Chemistry and Biochemistry, Utah State University Logan, Logan, UT 84322-0300, USA; [email protected] * Correspondence: [email protected] (W.Z.); [email protected] (M.M.) Abstract: Over the last years, scientific interest in noncovalent interactions based on the presence of electron-depleted regions called σ-holes or π-holes has markedly accelerated. Their high directionality and strength, comparable to hydrogen bonds, has been documented in many fields of modern chemistry. The current review gathers and digests recent results concerning these bonds, with a focus on those systems where both σ and π-holes are present on the same molecule. The underlying principles guiding the bonding in both sorts of interactions are discussed, and the trends that emerge from recent work offer a guide as to how one might design systems that allow multiple noncovalent bonds to occur simultaneously, or that prefer one bond type over another. Keywords: molecular electrostatic potential; halogen bond; pnicogen bond; tetrel bond; chalcogen bond; cooperativity Citation: Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Noncovalent Bonds through Sigma and Pi-Hole Located on the Same 1. Introduction Molecule. Guiding Principles and The concept of the σ-hole, introduced to a wide audience in 2005 at a conference in Comparisons. Molecules 2021, 26, Prague by Tim Clark [1], influenced a way of thinking about noncovalent interactions 1740. -

No Rabbit Ears on Water. the Structure of the Water Molecule
No Rabbit Ears on Water The Structure of the Water Molecule: What Should We Tell the Students? Michael Laing University of Natal. Durban, South Africa In the vast majority of high school (I)and freshman uni- He also considers the possibility of s character in the bond- versity (2) chemistry textbooks, water is presented as a bent ing orbitals of OF2 and OH2 to improve the "strength" of the molecule whose bonding can be described bv the Gilles~ie- orbital (p 111) from 1.732 for purep to 2.00 for the ideal sp3. Nyholm approach with the oxygen atomsp3 Gyhridized. The Inclusion of s character in the hybrid will be "resisted in the lone uairs are said to he dominant causine the H-O-H anele case of atoms with an nnshared pair.. in the s orbital, to beieduced from the ideal 1091120to 104' and are regul&ly which is more stable than the p orbitals" (p 120). Estimates shown as equivalent in shape and size even being described of the H-E-H angle range from 91.2 for AsH3 to 93.5O for as "rabbit ears" (3). Chemists have even been advised to Hz0. He once again ascribes the anomalies for Hz0 to "re- wear Mickey Mouse ears when teaching the structure and uulsion of the charzes of the hvdroaeu atoms" (D 122). bonding of the H20molecule, presumably to emphasize the - In his famous hook (6)~itac~oro&kii describks the bond- shape of the molecule (and possibly the two equivalent lone ing in water and HzS (p 10). -

The Hydrogen Bond: a Hundred Years and Counting
J. Indian Inst. Sci. A Multidisciplinary Reviews Journal ISSN: 0970-4140 Coden-JIISAD © Indian Institute of Science 2019. The Hydrogen Bond: A Hundred Years and Counting Steve Scheiner* REVIEW REVIEW ARTICLE Abstract | Since its original inception, a great deal has been learned about the nature, properties, and applications of the H-bond. This review summarizes some of the unexpected paths that inquiry into this phenom- enon has taken researchers. The transfer of the bridging proton from one molecule to another can occur not only in the ground electronic state, but also in various excited states. Study of the latter process has devel- oped insights into the relationships between the nature of the state, the strength of the H-bond, and the height of the transfer barrier. The enormous broadening of the range of atoms that can act as both pro- ton donor and acceptor has led to the concept of the CH O HB, whose ··· properties are of immense importance in biomolecular structure and function. The idea that the central bridging proton can be replaced by any of various electronegative atoms has fostered the rapidly growing exploration of related noncovalent bonds that include halogen, chalco- gen, pnicogen, and tetrel bonds. Keywords: Halogen bond, Chalcogen bond, Pnicogen bond, Tetrel bond, Excited-state proton transfer, CH O H-bond ··· 1 Introduction charge on the proton. The latter can thus attract Over the course of its century of study following the partial negative charge of an approaching its earliest conceptual formulation1,2, the hydro- nucleophile D. Another important factor resides gen bond (HB) has surrendered many of the in the perturbation of the electronic structure mysteries of its source of stability and its myriad of the two species as they approach one another. -

Chemical Physics 524 (2019) 55–62
Chemical Physics 524 (2019) 55–62 Contents lists available at ScienceDirect Chemical Physics journal homepage: www.elsevier.com/locate/chemphys Structures of clusters surrounding ions stabilized by hydrogen, halogen, T chalcogen, and pnicogen bonds ⁎ ⁎ Steve Scheinera, , Mariusz Michalczykb, Wiktor Zierkiewiczb, a Department of Chemistry and Biochemistry, Utah State University Logan, Utah 84322-0300, United States b Faculty of Chemistry, Wrocław University of Science and Technology, Wybrzeże Wyspiańskiego 27, 50-370 Wrocław, Poland ARTICLE INFO ABSTRACT Keywords: Four H-binding HCl and HF molecules position themselves at the vertices of a tetrahedron when surrounding a Tetrahedral central Cl−. Halogen bonding BrF and ClF form a slightly distorted tetrahedron, a tendency that is amplified for QTAIM ClCN which forms a trigonal pyramid. Chalcogen bonding SF2, SeF2, SeFMe, and SeCSe occupy one hemisphere NBO of the central ion, leaving the other hemisphere empty. This pattern is repeated for pnicogen bonding PF3, SeF3 Cl− and AsCF. The clustering of solvent molecules on one side of the Cl− is attributed to weak attractive interactions Na+ between them, including chalcogen, pnicogen, halogen, and hydrogen bonds. Binding energies of four solvent molecules around a central Na+ are considerably reduced relative to chloride, and the geometries are different, with no empty hemisphere. The driving force maximizes the number of electronegative (F or O) atoms close to the Na+, and the presence of noncovalent bonds between solvent molecules. 1. Introduction particular, the S, Se, etc group can engage in chalcogen bonds (YBs) [19–25], and the same is true of the P, As family which forms pnicogen The hydrogen bond (HB) is arguably the most important and in- bonds (ZBs) [26–34]. -

Quantitative Analysis of Hydrogen and Chalcogen Bonds in Two Pyrimidine
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Quantitative analysis of hydrogen and chalcogen bonds in two pyrimidine-5-carbonitrile derivatives, potential DHFR inhibitors: an integrated crystallographic and theoretical study Al-Wahaibi, Lamya H ; Chakraborty, Kushumita ; Al-Shaalan, Nora H ; Syed Majeed, Mohamed Yehya Annavi ; Blacque, Olivier ; Al-Mutairi, Aamal A ; El-Emam, Ali A ; Percino, M Judith ; Thamotharan, Subbiah Abstract: Two potential bioactive pyrimidine-5-carbonitrile derivatives have been synthesized and char- acterized by spectroscopic techniques (1H and 13C-NMR) and the three dimensional structures were elucidated by single crystal X-ray diffraction at low temperature (160 K). In both structures, the molec- ular conformation is locked by an intramolecular C–HC interaction involving the cyano and CH of the thiophene and phenyl rings. The intermolecular interactions were analyzed in a qualitative man- ner based on the Hirshfeld surface and 2D-fingerprint plots. The results suggest that the phenyl and thiophene moieties have an effect on the crystal packing. For instance, the chalcogen bonds areonly preferred in the thiophene derivative. However, both structures uses a common N–HO hydrogen bond motif. Moreover, the structures of 1 and 2 display 1D isostructurality and molecular chains stabilize by intermolecular N–HO and N–HN hydrogen bonds. The nature and extent of different non-covalent interactions were further characterized by the topological parameters derived from the quantum the- ory of atoms-in-molecules approach. This analysis indicates that apart from N–HO hydrogen bonds, other non-covalent interactions are closed-shell in nature. -
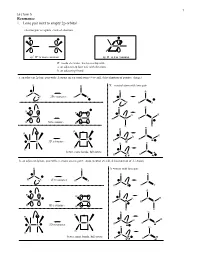
Resonance 1. Lone Pair Next to Empty 2P Orbital
1 Lecture 5 Resonance 1. Lone pair next to empty 2p orbital electron pair acceptors - lack of electrons C C CC sp2 R+ is more common sp R+ is less common R+ needs electrons, has to overlap with a. an adjacent 2p lone pair with electrons b. an adjacent pi bond a. an adjacent 2p lone pair with electrons on a neutral atom (+ overall, delocalization of positive charge) R R X = neutral atom with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R N R N R 3D resonance R R R R R R R C R C R CX R O CX 3D resonance R O R R R R C better, more bonds, full octets C R F R F b. an adjacent 2p lone pair with electrons on a negative atom (neutral overall, delocalization of electrons) R R X =anion with lone pair R R C C R X 2D resonance R X C C R X R X R R R R C R C R CX CX R C R C R 3D resonance R R R R R R R C R C R CX R N CX 3D resonance R N R R R R C C better, more bonds, full octets R O R O 2 Lecture 5 Problem 1 – All of the following examples demonstrate delocalization of a lone pair of electrons into an empty 2p orbital. Usually in organic chemistry this is a carbocation site, but not always. -

Effect of Lone Pairs on Hybridization
EFFECT OF LONE PAIRS ON HYBRIDIZATION We have discussed the effect of ghost lone pairs on the shape of a molecule in the post about VSEPR theory. The effect lone pairs exert is the same in VBT as it was in VSEPR. In this post we will study a few examples in which central atom possess lone pairs. First, we will take the example of NH3 molecule. When you draw its Lewis dot structure, you will find three bonding pairs of electrons and one lone pair on N. Now write the ground state configuration of N. 7N: 1s2, 2s2, 2p3 N already has 3 unpaired electrons in ground state to make bond with 3 H atoms. You may think why does N need hybridization in this case when it has 3 unpaired electrons of the same orbital p? Because hybridized orbitals are more competent in overlapping which results in a stronger bond. 3 In NH3 molecule, N chooses sp hybridization. This may get you puzzled again, if N has 3 unpaired electrons in p why does it include s orbital which has paired electrons? You know that the atoms follow Hund’s rule for filling electrons in orbitals, similarly they have to consider energy order of orbitals in hybridization also. When an atom undergoes hybridization, it has to pick orbitals in the increasing order of their energies. In NH3, N has to first pick 2s then 2px, 2py and then 2pz. N can’t ignore 2s even if it is filled because 2s has the lowest energy and N has to include all three 2p orbitals because it needs them for bonding with three H atoms. -

How Do We Know That There Are Atoms
Chemistry 11 Fall 2010 Discussion – 12/13/10 MOs and Hybridization 1. a. [:C N:]- Bond order = (8 – 2) / 2 = 3 *2p * 2p C 2p N 2p 2p 2p *2s C 2s N 2s 2s CN- b. Comparison of Lewis and MO models of CN- CN- Consistent: Both have triple bond (B.O. = 3) Inconsistent: - Lewis structure shows lone pairs on each atom rather than all e- being shared (MO) - Lewis structure indicates a negative formal charge on C, whereas MO suggests more electron density on N, which is consistent with EN considerations c. Both molecules exhibit sp mixing, and the MOs should reflect this. In N2, the molecular orbitals would be distributed equally between the two N atoms, whereas in CN-, the bonding orbitals have greater electron density on the more electronegative atom (N). The antibonding orbitals have more electron density on the less electronegative atom (C). - 1 - Chemistry 11 Fall 2010 2. a. Completed structure of guanine (carbons at each intersection are implied): * * * * b. 17 sigma bonds; 4 pi bonds. c. All carbons are sp2 hybridized; trigonal planar; with 120˚ bond angles. d. We predict that the three N’s marked with * are sp3 hybridized, with 109.5˚ bond angles. The remaining N atoms are sp2 hybridized, with 120˚ bond angles. e. i. Benzene: ii. For the structure drawn in (a), the bond angles in the six membered ring would be expected to be 120˚ for each atom except for the N§, which is predicted to have bond angles of 109.5˚. However, it is not possible to form a planar six-membered ring unless ALL the angles are 120˚. -

Frontiers in Halogen and Chalcogen-Bond Donor
DOI: 10.1002/cctc.201901215 Minireviews 1 2 3 Frontiers in Halogen and Chalcogen-Bond Donor 4 5 Organocatalysis 6 [a] [a] [a] 7 Julia Bamberger, Florian Ostler, and Olga García Mancheño* 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 ChemCatChem 2019, 11, 1–15 1 © 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim These are not the final page numbers! �� Wiley VCH Mittwoch, 21.08.2019 1999 / 145245 [S. 1/15] 1 Minireviews 1 Non-covalent molecular interactions on the basis of halogen Being particularly relevant in the binding of “soft” substrates, 2 and chalcogen bonding represent a promising, powerful the similar strength to hydrogen bonding interactions and its 3 catalytic activation mode. However, these “unusual” non- higher directionality allows for solution-phase applications with 4 covalent interactions are typically employed in the solid state halogen and chalcogen bonding as the key interaction. In this 5 and scarcely exploited in catalysis. In recent years, an increased mini-review, the special features, state-of-the-art and key 6 interest in halogen and chalcogen bonding have been awaken, examples of these so-called σ-hole interactions in the field of 7 as they provide profound characteristics that make them an organocatalysis are presented. 8 appealing alternative to the well-explored hydrogen bonding. 9 10 1. Introduction including crystal engineering,[8] supramolecular[9] and medicinal 11 chemistry.[8a,10] Besides the correlation to the electrostatic 12 To achieve an effective catalytic transformation, the structural potential, also charge transfer and dispersion is believed to give 13 design of selective catalyst structures requires the correct rise to σ-hole interactions.[11] Associated with σ* orbitals, the σ- 14 manipulation of the energetic and stereochemical features of hole deepens with heavier atoms (going from the top to the 15 intermolecular forces. -

Localized Electron Model for Bonding What Does Hybridization Mean?
190 Localized electron model for bonding 1. Determine the Lewis Structure for the molecule 2. Determine resonance structures 3. Determine the shape of the molecule 4. Determine the hybridization of the atoms What does hybridization mean? Why hybridize? Look at the shape of many molecules, and compare this to the shape of the orbitals of each of the atoms. Shape of orbitals Orbitals on atoms are s, p, d, and f, and there are certain shapes associated with each orbital. an s orbital is a big sphere the f orbitals look like two four leaf the p orbitals looks like “8” ’s clovers each one points in along an axis three f orbitals look like weird “8”’s with two doughnuts around each four of the d orbitals look like four one leaf clovers one d orbital looks like an “8” with a doughnut around the middle Shape of molecules Remember we determine the shape of molecules by determining the number of sets of electrons around the atom. count double and triple bonds as one set, count single bonds as one set of electrons and count lone pairs as a set 2 sets—each pair of electrons points 180° away from the other 5 sets—each pair points toward the corner of a trigonal bipyramid 3 sets—each pair points toward the corner of a triangle 6 sets—each pair points toward the corners of an octahedron 4 sets—each pair points toward the corner of a tetrahedron 191 To make bonds we need orbitals—the electrons need to go some where. -

Origin of Chalcogen-Bonding Interactions
Edinburgh Research Explorer The Origin of Chalcogen-Bonding Interactions Citation for published version: Pascoe, D, Ling, K & Cockroft, S 2017, 'The Origin of Chalcogen-Bonding Interactions', Journal of the American Chemical Society, vol. 139, no. 42, pp. 15160–15167. https://doi.org/10.1021/jacs.7b08511 Digital Object Identifier (DOI): 10.1021/jacs.7b08511 Link: Link to publication record in Edinburgh Research Explorer Document Version: Peer reviewed version Published In: Journal of the American Chemical Society General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 11. Oct. 2021 The Origin of Chalcogen-Bonding Interactions Dominic J. Pascoe†, Kenneth B. Ling‡, and Scott L. Cockroft†* †EaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, EH9 3FJ, UK ‡Syngenta, Jealott’s Hill International Research Centre, Bracknell, Berkshire, RG42 6EY, UK ABSTRACT: Favorable molecular interactions between group 16 elements have been implicated in catalysis, biological processes, materials and medicinal chemistry. Such interactions have since become known as chalcogen bonds by analogy to hydrogen and halogen bonds. -

Theoretical Study on the Noncovalent Interactions Involving Triplet Diphenylcarbene
Theoretical Study on the Noncovalent Interactions Involving Triplet Diphenylcarbene Chunhong Zhao Hebei Normal University Huihua College Hui Lin Hebei Normal University Aiting Shan Hebei Normal University Shaofu Guo Hebei Normal University Huihua College Xiaoyan Li Hebei Normal University Xueying Zhang ( [email protected] ) Hebei Normal University https://orcid.org/0000-0003-1598-8501 Research Article Keywords: triplet diphenylcarbene, noncovalent interaction, electron density shift, electron spin density Posted Date: May 19th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-445417/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Journal of Molecular Modeling on July 9th, 2021. See the published version at https://doi.org/10.1007/s00894-021-04838-6. Page 1/21 Abstract The properties of some types of noncovalent interactions formed by triplet diphenylcarbene (DPC3) have been investigated by means of density functional theory (DFT) calculations and quantum theory of 3 atoms in molecules (QTAIM) studies. The DPC ···LA (LA = AlF3, SiF4, PF5, SF2, ClF) complexes have been analyzed from their equilibrium geometries, binding energies, charge transfer and properties of electron 3 density. The triel bond in the DPC ···AlF3 complex exhibits a partially covalent nature, with the binding energy − 65.7kJ/mol. The tetrel bond, pnicogen bond, chalcogen bond and halogen bond in the DPC3···LA (LA = SiF4, PF5, SF2, ClF) complexes show the character of a weak closed-shell noncovalent interaction. Polarization plays an important role in the formation of the studied complexes.