Determining the Extent of Hothouse Climate Effects on the Jurassic Silica Cycle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1. Shatsky Rise: Seismic Stratigraphy and Sedimentary Record of Pacific Paleoceanography Since the Early Cretaceous1
Natland, J.H., Storms, M.A., et al., 1993 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 132 1. SHATSKY RISE: SEISMIC STRATIGRAPHY AND SEDIMENTARY RECORD OF PACIFIC PALEOCEANOGRAPHY SINCE THE EARLY CRETACEOUS1 William V. Sliter2 and Glenn R. Brown3 ABSTRACT Shatsky Rise consists of three highs arranged in a linear trend more than 1300 km long. Shatsky Plateau, the southernmost and largest of three highs is represented by an exposed basement high of presumed Late Jurassic age flanked by a sedimentary sequence of at least Cretaceous and Cenozoic age that reaches a maximum thickness of more than 1100 m. Drilling on Shatsky Rise is restricted to eight DSDP and ODP sites on the southern plateau that partially penetrated the sedimentary sequence. Leg 132 seismic profiles and previous seismic records from Shatsky Plateau reveal a five-part seismic section that is correlated with the drilling record and used to interpret the sedimentary history of the rise. The seismic sequence documents the transit of Shatsky Plateau beneath the equatorial divergence in the Late Cretaceous by horizontal plate motion from an original location in the Southern Hemisphere. Unconformities and lithologic changes bounding several of the seismic units are correlated with pale- oceanographic changes that resulted in erosional events near the Barremian/Aptian, Cenomanian/Turonian, and Paleogene/Neo- gene boundaries. INTRODUCTION Plateau, is the largest with a length of about 700 km and a width of about 300 km. All previous DSDP and ODP drill sites are located on Shatsky Rise, the second largest oceanic plateau in the Pacific the southern plateau (Fig. -

Subsidence and Growth of Pacific Cretaceous Plateaus
ELSEVIER Earth and Planetary Science Letters 161 (1998) 85±100 Subsidence and growth of Paci®c Cretaceous plateaus Garrett Ito a,Ł, Peter D. Clift b a School of Ocean and Earth Science and Technology, POST 713, University of Hawaii at Manoa, Honolulu, HI 96822, USA b Department of Geology and Geophysics, Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA Received 10 November 1997; revised version received 11 May 1998; accepted 4 June 1998 Abstract The Ontong Java, Manihiki, and Shatsky oceanic plateaus are among the Earth's largest igneous provinces and are commonly believed to have erupted rapidly during the surfacing of giant heads of initiating mantle plumes. We investigate this hypothesis by using sediment descriptions of Deep Sea Drilling Project (DSDP) and Ocean Drilling Program (ODP) drill cores to constrain plateau subsidence histories which re¯ect mantle thermal and crustal accretionary processes. We ®nd that total plateau subsidence is comparable to that expected of normal sea¯oor but less than predictions of thermal models of hotspot-affected lithosphere. If crustal emplacement was rapid, then uncertainties in paleo-water depths allow for the anomalous subsidence predicted for plumes with only moderate temperature anomalies and volumes, comparable to the sources of modern-day hotspots such as Hawaii and Iceland. Rapid emplacement over a plume head of high temperature and volume, however, is dif®cult to reconcile with the subsidence reconstructions. An alternative possibility that reconciles low subsidence over a high-temperature, high-volume plume source is a scenario in which plateau subsidence is the superposition of (1) subsidence due to the cooling of the plume source, and (2) uplift due to prolonged crustal growth in the form of magmatic underplating. -

Calcareous Nannofossil Zonation and Sequence Stratigraphy of the Jurassic System, Onshore Kuwait
GeoArabia, 2015, v. 20, no. 4, p. 125-180 Gulf PetroLink, Bahrain Calcareous nannofossil zonation and sequence stratigraphy of the Jurassic System, onshore Kuwait Adi P. Kadar, Thomas De Keyser, Nilotpaul Neog and Khalaf A. Karam (with contributions from Yves-Michel Le Nindre and Roger B. Davies) ABSTRACT This paper presents the calcareous nannofossil zonation of the Middle and Upper Jurassic of onshore Kuwait and formalizes current stratigraphic nomenclature. It also interprets the positions of the Jurassic Arabian Plate maximum flooding surfaces (MFS J10 to J110 of Sharland et al., 2001) and sequence boundaries in Kuwait, and correlates them to those in central Saudi Arabia outcrops. This study integrates data from about 400 core samples from 11 wells representing a nearly complete Middle to Upper Jurassic stratigraphic succession. Forty-two nannofossil species were identified using optical microscope techniques. The assemblage contains Tethyan nannofossil markers, which allow application of the Jurassic Tethyan nannofossil biozones. Six zones and five subzones, ranging in age from Middle Aalenian to Kimmeridgian, are established using first and last occurrence events of diagnostic calcareous nannofossil species. A chronostratigraphy of the studied formations is presented, using the revised formal stratigraphic nomenclature. The Marrat Formation is barren of nannofossils. Based on previous studies it is dated as Late Sinemurian–Early Aalenian and contains Middle Toarcian MFS J10. The overlying Dhruma Formation is Middle or Late Aalenian (Zone NJT 8c) or older, to Late Bajocian (Subzone NJT 10a), and contains Lower Bajocian MFS J20. The overlying Sargelu Formation consists of the Late Bajocian (Subzone NJT 10b) Sargelu-Dhruma Transition, and mostly barren Sargelu Limestone in which we place Lower Bathonian MFS J30 near its base. -
Letter to the Editor
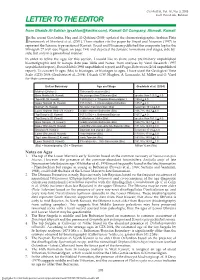
GeoArabia, Vol. 10, No. 3, 2005 Gulf PetroLink, Bahrain LETTER TO THE EDITOR from Ghaida Al-Sahlan ([email protected]), Kuwait Oil Company, Ahmadi, Kuwait n the recent GeoArabia, Haq and Al-Qahtani (2005) updated the chronostratigraphic Arabian Plate Iframework of Sharland et al. (2001). These studies cite the paper by Yousif and Nouman (1997) to represent the Jurassic type section of Kuwait. Yousif and Nouman published the composite log for the Minagish-27 well (see Figure on page 194) and depicted the Jurassic formations and stages, side-by- side, but only in a generalized manner. In order to refine the ages for this section, I would like to share some preliminary unpublished biostratigraphic and Sr isotope data (see Table and Notes) from analyses by Varol Research (1997 unpublished report), ExxonMobil (1998 unpublished report) and Fugro-Robertson (2004 unpublished report). To convert Sr ages (Ma) to biostages, or biostages to ages, I have used the Geological Time Scale (GTS) 2004 (Gradstein et al., 2004). I thank G.W. Hughes, A. Lomando, M. Miller and O. Varol for their comments. Unit or Boundary Age and Stage Gradstein et al. (2004) Makhul (Offshore) Tithonian-Berriasian (Bio) Base Makhul (N. Kuwait) No younger than Tithonian (Bio) greater than 145.5 + 4.0 Top Hith (W. Kuwait) 150.0 (Sr) = c. Tithonian/Kimmeridgian ? 150.8 + 4.0 Upper Najmah (S. Kuwait) 155.0 (Sr) = c. Kimmeridgian/Oxfordian 155.7 + 4.0 Najmah (N. Kuwait) No older than Oxfordian (Bio) less than 161.2 + 4.0 Lower Najmah Shale (N. Kuwait) middle and late Bathonian (Bio) 166.7 to 164.7 + 4.0 Top Sargelu (S. -

The Late Jurassic Tithonian, a Greenhouse Phase in the Middle Jurassic–Early Cretaceous ‘Cool’ Mode: Evidence from the Cyclic Adriatic Platform, Croatia
Sedimentology (2007) 54, 317–337 doi: 10.1111/j.1365-3091.2006.00837.x The Late Jurassic Tithonian, a greenhouse phase in the Middle Jurassic–Early Cretaceous ‘cool’ mode: evidence from the cyclic Adriatic Platform, Croatia ANTUN HUSINEC* and J. FRED READ *Croatian Geological Survey, Sachsova 2, HR-10000 Zagreb, Croatia Department of Geosciences, Virginia Tech, 4044 Derring Hall, Blacksburg, VA 24061, USA (E-mail: [email protected]) ABSTRACT Well-exposed Mesozoic sections of the Bahama-like Adriatic Platform along the Dalmatian coast (southern Croatia) reveal the detailed stacking patterns of cyclic facies within the rapidly subsiding Late Jurassic (Tithonian) shallow platform-interior (over 750 m thick, ca 5–6 Myr duration). Facies within parasequences include dasyclad-oncoid mudstone-wackestone-floatstone and skeletal-peloid wackestone-packstone (shallow lagoon), intraclast-peloid packstone and grainstone (shoal), radial-ooid grainstone (hypersaline shallow subtidal/intertidal shoals and ponds), lime mudstone (restricted lagoon), fenestral carbonates and microbial laminites (tidal flat). Parasequences in the overall transgressive Lower Tithonian sections are 1– 4Æ5 m thick, and dominated by subtidal facies, some of which are capped by very shallow-water grainstone-packstone or restricted lime mudstone; laminated tidal caps become common only towards the interior of the platform. Parasequences in the regressive Upper Tithonian are dominated by peritidal facies with distinctive basal oolite units and well-developed laminate caps. Maximum water depths of facies within parasequences (estimated from stratigraphic distance of the facies to the base of the tidal flat units capping parasequences) were generally <4 m, and facies show strongly overlapping depth ranges suggesting facies mosaics. Parasequences were formed by precessional (20 kyr) orbital forcing and form parasequence sets of 100 and 400 kyr eccentricity bundles. -

And Early Jurassic Sediments, and Patterns of the Triassic-Jurassic
and Early Jurassic sediments, and patterns of the Triassic-Jurassic PAUL E. OLSEN AND tetrapod transition HANS-DIETER SUES Introduction parent answer was that the supposed mass extinc- The Late Triassic-Early Jurassic boundary is fre- tions in the tetrapod record were largely an artifact quently cited as one of the thirteen or so episodes of incorrect or questionable biostratigraphic corre- of major extinctions that punctuate Phanerozoic his- lations. On reexamining the problem, we have come tory (Colbert 1958; Newell 1967; Hallam 1981; Raup to realize that the kinds of patterns revealed by look- and Sepkoski 1982, 1984). These times of apparent ing at the change in taxonomic composition through decimation stand out as one class of the great events time also profoundly depend on the taxonomic levels in the history of life. and the sampling intervals examined. We address Renewed interest in the pattern of mass ex- those problems in this chapter. We have now found tinctions through time has stimulated novel and com- that there does indeed appear to be some sort of prehensive attempts to relate these patterns to other extinction event, but it cannot be examined at the terrestrial and extraterrestrial phenomena (see usual coarse levels of resolution. It requires new fine- Chapter 24). The Triassic-Jurassic boundary takes scaled documentation of specific faunal and floral on special significance in this light. First, the faunal transitions. transitions have been cited as even greater in mag- Stratigraphic correlation of geographically dis- nitude than those of the Cretaceous or the Permian junct rocks and assemblages predetermines our per- (Colbert 1958; Hallam 1981; see also Chapter 24). -

Kimmeridgian (Late Jurassic) Cold-Water Idoceratids (Ammonoidea) from Southern Coahuila, Northeastern Mexico, Associated with Boreal Bivalves and Belemnites
REVISTA MEXICANA DE CIENCIAS GEOLÓGICAS Kimmeridgian cold-water idoceratids associated with Boreal bivalvesv. 32, núm. and 1, 2015, belemnites p. 11-20 Kimmeridgian (Late Jurassic) cold-water idoceratids (Ammonoidea) from southern Coahuila, northeastern Mexico, associated with Boreal bivalves and belemnites Patrick Zell* and Wolfgang Stinnesbeck Institute for Earth Sciences, Heidelberg University, Im Neuenheimer Feld 234, 69120 Heidelberg, Germany. *[email protected] ABSTRACT et al., 2001; Chumakov et al., 2014) was followed by a cool period during the late Oxfordian-early Kimmeridgian (e.g., Jenkyns et al., Here we present two early Kimmeridgian faunal assemblages 2002; Weissert and Erba, 2004) and a long-term gradual warming composed of the ammonite Idoceras (Idoceras pinonense n. sp. and trend towards the Jurassic-Cretaceous boundary (e.g., Abbink et al., I. inflatum Burckhardt, 1906), Boreal belemnites Cylindroteuthis 2001; Lécuyer et al., 2003; Gröcke et al., 2003; Zakharov et al., 2014). cuspidata Sachs and Nalnjaeva, 1964 and Cylindroteuthis ex. gr. Palynological data suggest that the latest Jurassic was also marked by jacutica Sachs and Nalnjaeva, 1964, as well as the Boreal bivalve Buchia significant fluctuations in paleotemperature and climate (e.g., Abbink concentrica (J. de C. Sowerby, 1827). The assemblages were discovered et al., 2001). in inner- to outer shelf sediments of the lower La Casita Formation Upper Jurassic-Lower Cretaceous marine associations contain- at Puerto Piñones, southern Coahuila, and suggest that some taxa of ing both Tethyan and Boreal elements [e.g. ammonites, belemnites Idoceras inhabited cold-water environments. (Cylindroteuthis) and bivalves (Buchia)], were described from numer- ous localities of the Western Cordillera belt from Alaska to California Key words: La Casita Formation, Kimmeridgian, idoceratid ammonites, (e.g., Jeletzky, 1965), while Boreal (Buchia) and even southern high Boreal bivalves, Boreal belemnites. -

Orbital Pacing and Secular Evolution of the Early Jurassic Carbon Cycle
Orbital pacing and secular evolution of the Early Jurassic carbon cycle Marisa S. Storma,b,1, Stephen P. Hesselboc,d, Hugh C. Jenkynsb, Micha Ruhlb,e, Clemens V. Ullmannc,d, Weimu Xub,f, Melanie J. Lengg,h, James B. Ridingg, and Olga Gorbanenkob aDepartment of Earth Sciences, Stellenbosch University, 7600 Stellenbosch, South Africa; bDepartment of Earth Sciences, University of Oxford, OX1 3AN Oxford, United Kingdom; cCamborne School of Mines, University of Exeter, TR10 9FE Penryn, United Kingdom; dEnvironment and Sustainability Institute, University of Exeter, TR10 9FE Penryn, United Kingdom; eDepartment of Geology, Trinity College Dublin, The University of Dublin, Dublin 2, Ireland; fDepartment of Botany, Trinity College Dublin, The University of Dublin, Dublin 2, Ireland; gEnvironmental Science Centre, British Geological Survey, NG12 5GG Nottingham, United Kingdom; and hSchool of Biosciences, University of Nottingham, LE12 5RD Loughborough, United Kingdom Edited by Lisa Tauxe, University of California San Diego, La Jolla, CA, and approved January 3, 2020 (received for review July 14, 2019) Global perturbations to the Early Jurassic environment (∼201 to where they are recorded as individual shifts or series of shifts ∼174 Ma), notably during the Triassic–Jurassic transition and Toar- within stratigraphically limited sections. Some of these short-term cian Oceanic Anoxic Event, are well studied and largely associated δ13C excursions have been shown to represent changes in the with volcanogenic greenhouse gas emissions released by large supraregional to global carbon cycle, marked by synchronous igneous provinces. The long-term secular evolution, timing, and changes in δ13C in marine and terrestrial organic and inorganic pacing of changes in the Early Jurassic carbon cycle that provide substrates and recorded on a wide geographic extent (e.g., refs. -

The Global Stratotype Sections and Point (GSSP) for the Base of the Jurassic System at Kuhjoch (Karwendel Mountains, Northern Calcareous Alps, Tyrol, Austria)
162 162 Articles by Hillebrandt, A.v.1, Krystyn, L.2, Kürschner, W.M.3, Bonis, N.R.4, Ruhl, M.5, Richoz, S.6, Schobben, M. A. N.12, Urlichs, M.7, Bown, P.R.8, Kment, K.9, McRoberts, C.A.10, Simms, M.11, and Tomãsových, A13 The Global Stratotype Sections and Point (GSSP) for the base of the Jurassic System at Kuhjoch (Karwendel Mountains, Northern Calcareous Alps, Tyrol, Austria) 1 Institut für Angewandte Geowissenschaften, Technische Universität, Ernst-Reuter-Platz 1, 10587 Berlin, Germany. E-mail: [email protected] 2 Department for Palaeontology, Vienna University, Geozentrum, Althansstr. 9, A-1090 Vienna, Austria. E-mail: [email protected] 3 Department of Geosciences and Centre of Earth Evolution and Dynamics (CEED), University of Oslo, PO box 1047, Blindern, 0316 Oslo, Norway. E-mail: [email protected] 4 Shell Global Solutions International B.V., Kessler Park 1, 2288 GS, Rijswijk, the Netherlands. E-mail: [email protected] 5 Department of Earth Sciences, University of Oxford, South Parks Road, Oxford OX1 3AN, UK. E-mail: [email protected] 6 Commission for the Palaeontological and Stratigraphical Research of Austria, Austrian Academy of Sciences c/o Institut of Earth Sciences, Graz University, Heinrichstraße 26, 8010 Graz, Austria. E-mail: [email protected] 7 Staatliches Museum für Naturkunde, Rosenstein 1, 70191 Stuttgart, Germany. E-mail: [email protected] 8 Department of Earth Sciences, University College London, Gower Street, London WC1E 6BT, UK. E-mail: [email protected] 9 Lenggrieser Str. -

Geochemistry and Age of Shatsky, Hess, and Ojin Rise Seamounts: Implications for a Connection Between the Shatsky and Hess Rises
Accepted Manuscript Geochemistry and Age of Shatsky, Hess, and Ojin Rise seamounts: Implications for a connection between the Shatsky and Hess Rises Maria Luisa G. Tejada, Jörg Geldmacher, Folkmar Hauff, Daniel Heaton, Anthony A.P. Koppers, Dieter Garbe-Schönberg, Kaj Hoernle, Ken Heydolph, William W. Sager PII: S0016-7037(16)30165-X DOI: http://dx.doi.org/10.1016/j.gca.2016.04.006 Reference: GCA 9701 To appear in: Geochimica et Cosmochimica Acta Received Date: 4 September 2015 Accepted Date: 1 April 2016 Please cite this article as: Tejada, M.L.G., Geldmacher, J., Hauff, F., Heaton, D., Koppers, A.A.P., Garbe- Schönberg, D., Hoernle, K., Heydolph, K., Sager, W.W., Geochemistry and Age of Shatsky, Hess, and Ojin Rise seamounts: Implications for a connection between the Shatsky and Hess Rises, Geochimica et Cosmochimica Acta (2016), doi: http://dx.doi.org/10.1016/j.gca.2016.04.006 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 Geochemistry and Age of Shatsky, Hess, and Ojin Rise seamounts: Implications 2 for a connection between the Shatsky and Hess Rises 3 Maria Luisa G. Tejada a,b*, Jörg Geldmacher c, Folkmar Hauff c, Daniel Heaton d, Anthony A. -

Upper Bajocian– Callovian) of the Polish Jura Chain and Holy Cross Mountains (South-Central Poland)
1661-8726/07/010153-12 Swiss j. geosci. 100 (2007) 153–164 DOI 10.1007/s00015-007-1207-3 Birkhäuser Verlag, Basel, 2007 A diverse crinoid fauna from the Middle Jurassic (Upper Bajocian– Callovian) of the Polish Jura Chain and Holy Cross Mountains (south-central Poland) MARIUSZ A. SALAMON*& MICHA¸ ZATO¡ Key words: crinoids, Middle Jurassic, Poland, palaeobiogeography, taphonomy, epibiontism ABSTRACT ZUSAMMENFASSUNG A systematic account of a diverse crinoid fauna from the Middle Jurassic Aus mitteljurassischen (Bajocian–Callovian) Sedimenten des südlichen (Upper Bajocian–Callovian) of the Polish Jura Chain and Holy Cross Moun- Zentralpolens (Krakow–Cz´stochowa Hochland und Heilig-Kreuz Gebirge) tains (south-central Poland) is presented. The description is supplemented wird eine diverse Crinoidenfauna systematisch beschrieben und stratigra- with a list of all crinoid species found hitherto in the Tatra Mountains and the phisch eingestuft. Die Beschreibung wird durch eine Zusammenstellung sämt- Pieniny Klippen Belt (Poland), which were a part of the northern margin of licher Crinoiden-Spezies ergänzt, die bislang im Tatra-Gebirge und im Pieniny the Tethys during Middle Jurassic time. Balanocrinus hessi seems to be en- Klippen-Gürtel gefunden wurden. Beide Regionen waren während des Mitt- demic and established its own population in the epicontinental sea. Other leren Jura Teil des Nordrandes der Tethys. Balanocrinus hessi bildete eigen- stalked crinoids entered from the Tethys through the East-Carpathian Gate or ständige Populationen in diesem epikontinentalen Meeresbereich und scheint from a westerly way, and constitute a typical Mediterranean fauna. Stemless endemisch gewesen zu sein. Andere gestielte Crinoiden drangen aus der forms are regarded to be unsuccessful immigrants. -

Connecting the Deep Earth and the Atmosphere
In Mantle Convection and Surface Expression (Cottaar, S. et al., eds.) AGU Monograph 2020 (in press) Connecting the Deep Earth and the Atmosphere Trond H. Torsvik1,2, Henrik H. Svensen1, Bernhard Steinberger3,1, Dana L. Royer4, Dougal A. Jerram1,5,6, Morgan T. Jones1 & Mathew Domeier1 1Centre for Earth Evolution and Dynamics (CEED), University of Oslo, 0315 Oslo, Norway; 2School of Geosciences, University of Witwatersrand, Johannesburg 2050, South Africa; 3Helmholtz Centre Potsdam, GFZ, Telegrafenberg, 14473 Potsdam, Germany; 4Department of Earth and Environmental Sciences, Wesleyan University, Middletown, Connecticut 06459, USA; 5DougalEARTH Ltd.1, Solihull, UK; 6Visiting Fellow, Earth, Environmental and Biological Sciences, Queensland University of Technology, Brisbane, Queensland, Australia. Abstract Most hotspots, kimberlites, and large igneous provinces (LIPs) are sourced by plumes that rise from the margins of two large low shear-wave velocity provinces in the lowermost mantle. These thermochemical provinces have likely been quasi-stable for hundreds of millions, perhaps billions of years, and plume heads rise through the mantle in about 30 Myr or less. LIPs provide a direct link between the deep Earth and the atmosphere but environmental consequences depend on both their volumes and the composition of the crustal rocks they are emplaced through. LIP activity can alter the plate tectonic setting by creating and modifying plate boundaries and hence changing the paleogeography and its long-term forcing on climate. Extensive blankets of LIP-lava on the Earth’s surface can also enhance silicate weathering and potentially lead to CO2 drawdown (cooling), but we find no clear relationship between LIPs and post-emplacement variation in atmospheric CO2 proxies on very long (>10 Myrs) time- scales.