Revising the Recent Evolutionary History of Equids Using Ancient DNA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Three-Toed Browsing Horse Anchitherium (Equidae) from the Miocene of Panama
J. Paleonl., 83(3), 2009, pp. 489-492 Copyright © 2009, The Paleontological Society 0022-3360/09/0083-489S03.00 THREE-TOED BROWSING HORSE ANCHITHERIUM (EQUIDAE) FROM THE MIOCENE OF PANAMA BRUCE J. MACFADDEN Florida Museum of Natural History, University of Florida, Gainesville FL 32611, <[email protected]> INTRODUCTION (CRNHT/APL); L, left; M, upper molar; R upper premolar; R, DURING THE Cenozoic, the New World tropics supported a rich right; TRN, greatest transverse width. biodiversity of mammals. However, because of the dense SYSTEMATIC PALEONTOLOGY vegetative ground cover, today relatively little is known about extinct mammals from this region (MacFadden, 2006a). In an Class MAMMALIA Linnaeus, 1758 exception to this generalization, fossil vertebrates have been col- Order PERISSODACTYLA Owen, 1848 lected since the second half of the twentieth century from Neo- Family EQUIDAE Gray, 1821 gene exposures along the Panama Canal. Whitmore and Stewart Genus ANCHITHERIUM Meyer, 1844 (1965) briefly reported on the extinct land mammals collected ANCHITHERIUM CLARENCI Simpson, 1932 from the Miocene Cucaracha Formation that crops out in the Gail- Figures 1, 2, Table 1 lard Cut along the southern reaches of the Canal. MacFadden Referred specimen.—UF 236937, partial palate (maxilla) with (2006b) formally described this assemblage, referred to as the L P1-M3, R P1-P3, and small fragment of anterointernal part of Gaillard Cut Local Fauna (L.E, e.g., Tedford et al., 2004), which P4 (Fig. 1). Collected by Aldo Rincon of the Smithsonian Tropical consists of at least 10 species of carnivores, artiodactyls (also see Research Institute, Republic of Panama, on 15 May 2008. -

Correlation Structure of the Cheek Teeth Enamel Crown Patterns in the Genus Equus (Mammalia: Equidae): an Analysis by Geometric Morphometrics with Outline Points
Russian J. Theriol. 20(1): 70–81 © RUSSIAN JOURNAL OF THERIOLOGY, 2021 Correlation structure of the cheek teeth enamel crown patterns in the genus Equus (Mammalia: Equidae): an analysis by geometric morphometrics with outline points Igor Ya. Pavlinov*, Natalia N. Spasskаya ABSTRACT. Correlation structure of the cheek teeth enamel crown patterns in the genus Equus was studied by means of geometric morphometrics using outline points as descriptors to reveal the levels of morpholog- ical integration of the toothrow elements. Crown patterns in 34 upper and 31 lower toothrows (260 teeth in total) from 30 horse species were analyzed, the respective sets of 70 to 150 outline points were processed using the elliptic Fourier, principal component, and cluster analyses. The most correlated were shown to be the serial homologous crown elements within premolar and molar toothrow portions and less across the total toothrow. Correlation between occluding upper and lower teeth was shown to be low. Such correlation structure allowed identifying several levels of integration of the cheek teeth crown patterns in the genus Equus. A possibility of considering the serial homologous crown elements as the modules of the evolutionary developmental structure of the equine toothrows was discussed. Certain perspectives of similar studies in the specialized artiodactyles were emphasized. How to cite this article: Pavlinov I.Ya., Spasskаya N.N. 2021. Correlation structure of the cheek teeth enamel crown patterns in the genus Equus (Mammalia: Equidae): an analysis by geometric morphometrics with outline points // Russian J. Theriol. Vol.20. No.1. P.70–81. doi: 10.15298/rusjtheriol.20.1.08. KEY WORDS: dentition, geometric morphometrics, outline points, Equus, levels of integration, evo-devo. -

Unit-V Evolution of Horse
UNIT-V EVOLUTION OF HORSE Horses (Equus) are odd-toed hooped mammals belong- ing to the order Perissodactyla. Horse evolution is a straight line evolution and is a suitable example for orthogenesis. It started from Eocene period. The entire evolutionary sequence of horse history is recorded in North America. " Place of Origin The place of origin of horse is North America. From here, horses migrated to Europe and Asia. By the end of Pleis- tocene period, horses became extinct in the motherland (N. America). The horses now living in N. America are the de- scendants of migrants from other continents. Time of Origin The horse evolution started some 58 million years ago, m the beginning of Eocene period of Coenozoic era. The modem horse Equus originated in Pleistocene period about 2 million years ago. Evolutionary Trends The fossils of horses that lived in different periods, show that the body parts exhibited progressive changes towards a particular direction. These directional changes are called evo- lutionary trends. The evolutionary trends of horse evolution are summarized below: 1. Increase in size. 2. Increase in the length of limbs. 3. Increase in the length of the neck. 4. Increase in the length of preorbital region (face). 5. Increase in the length and size of III digit. 6. Increase in the size and complexity of brain. 7. Molarization of premolars. Olfactory bulb Hyracotherium Mesohippus Equus Fig.: Evolution of brain in horse. 8. Development of high crowns in premolars and molars. 9. Change of plantigrade gait to unguligrade gait. 10. Formation of diastema. 11. Disappearance of lateral digits. -

The Last Populations of the Critically Endangered Onager Equus Hemionus Onager in Iran: Urgent Requirements for Protection and Study
Oryx Vol 37 No 4 October 2003 Short Communication The last populations of the Critically Endangered onager Equus hemionus onager in Iran: urgent requirements for protection and study Laurent Tatin, Bijan F. Darreh-Shoori, Christophe Tourenq, David Tatin and Bijan Azmayesh Abstract The onager Equus hemionus onager, a wild ass for domestic use, and land conversion have been identi- endemic to Iran, is categorized as Critically Endangered fied as the main threats to the two remaining onager on the IUCN Red List. Its biology and conservation populations. In addition, geographical isolation could requirements are poorly documented. We report our cause the loss of genetic variability in these two relatively observations, made in 1997 and 2000, on the behaviour small populations, and also makes them more susceptible and ecology of the two remaining populations, located to the potential eCects of stochastic events such as drought in the Touran Protected Area and the Bahram-e-Goor or disease. Public awareness, appropriate protection, and Reserve. Recent population counts by the Department of scientific studies must be urgently supported by both Environment of Iran (471 in the Protected Area and 96 national and international organizations in order to pre- in the Reserve) are markedly lower than the estimate of vent the extinction of these two apparently dwindling 600–770 made in the 1970s in the Touran Protected Area. populations of onager. We observed social interactions between stallions and mares outside the breeding season that contrasts with Keywords Ass, behaviour, conservation status, Equus the known social structure of this subspecies. Poaching, hemionus, Iran, onager. competition with domestic animals, removal of shrubs The Asiatic wild ass Equus hemionus is one of seven ass Equus h. -

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando
Genomics and the Evolutionary History of Equids Pablo Librado, Ludovic Orlando To cite this version: Pablo Librado, Ludovic Orlando. Genomics and the Evolutionary History of Equids. Annual Review of Animal Biosciences, Annual Reviews, 2021, 9 (1), 10.1146/annurev-animal-061220-023118. hal- 03030307 HAL Id: hal-03030307 https://hal.archives-ouvertes.fr/hal-03030307 Submitted on 30 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Annu. Rev. Anim. Biosci. 2021. 9:X–X https://doi.org/10.1146/annurev-animal-061220-023118 Copyright © 2021 by Annual Reviews. All rights reserved Librado Orlando www.annualreviews.org Equid Genomics and Evolution Genomics and the Evolutionary History of Equids Pablo Librado and Ludovic Orlando Laboratoire d’Anthropobiologie Moléculaire et d’Imagerie de Synthèse, CNRS UMR 5288, Université Paul Sabatier, Toulouse 31000, France; email: [email protected] Keywords equid, horse, evolution, donkey, ancient DNA, population genomics Abstract The equid family contains only one single extant genus, Equus, including seven living species grouped into horses on the one hand and zebras and asses on the other. In contrast, the equine fossil record shows that an extraordinarily richer diversity existed in the past and provides multiple examples of a highly dynamic evolution punctuated by several waves of explosive radiations and extinctions, cross-continental migrations, and local adaptations. -

Barren Ridge FEIS-Volume IV Paleo Tech Rpt Final March
March 2011 BARREN RIDGE RENEWABLE TRANSMISSION PROJECT Paleontological Resources Assessment Report PROJECT NUMBER: 115244 PROJECT CONTACT: MIKE STRAND EMAIL: [email protected] PHONE: 714-507-2710 POWER ENGINEERS, INC. PALEONTOLOGICAL RESOURCES ASSESSMENT REPORT Paleontological Resources Assessment Report PREPARED FOR: LOS ANGELES DEPARTMENT OF WATER AND POWER 111 NORTH HOPE STREET LOS ANGELES, CA 90012 PREPARED BY: POWER ENGINEERS, INC. 731 EAST BALL ROAD, SUITE 100 ANAHEIM, CA 92805 DEPARTMENT OF PALEOSERVICES SAN DIEGO NATURAL HISTORY MUSEUM PO BOX 121390 SAN DIEGO, CA 92112 ANA 032-030 (PER-02) LADWP (MARCH 2011) SB 115244 POWER ENGINEERS, INC. PALEONTOLOGICAL RESOURCES ASSESSMENT REPORT TABLE OF CONTENTS 1.0 INTRODUCTION ........................................................................................................................... 1 1.1 STUDY PERSONNEL ....................................................................................................................... 2 1.2 PROJECT DESCRIPTION .................................................................................................................. 2 1.2.1 Construction of New 230 kV Double-Circuit Transmission Line ........................................ 4 1.2.2 Addition of New 230 kV Circuit ......................................................................................... 14 1.2.3 Reconductoring of Existing Transmission Line .................................................................. 14 1.2.4 Construction of New Switching Station ............................................................................. -

Redalyc.Study of Cedral Horses and Their Place in the Mexican Quaternary
Revista Mexicana de Ciencias Geológicas ISSN: 1026-8774 [email protected] Universidad Nacional Autónoma de México México Alberdi, María Teresa; Arroyo-Cabrales, Joaquín; Marín-Leyva, Alejandro H.; Polaco, Oscar J. Study of Cedral Horses and their place in the Mexican Quaternary Revista Mexicana de Ciencias Geológicas, vol. 31, núm. 2, 2014, pp. 221-237 Universidad Nacional Autónoma de México Querétaro, México Available in: http://www.redalyc.org/articulo.oa?id=57231524006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative REVISTA MEXICANA DE CIENCIAS GEOLÓGICAS v. 31, núm. 2, 2014,Cedral p. 221-237horses Study of Cedral Horses and their place in the Mexican Quaternary María Teresa Alberdi1, Joaquín Arroyo-Cabrales2, Alejandro H. Marín-Leyva3, and Oscar J. Polaco2† 1 Departamento de Paleobiología, Museo Nacional de Ciencias Naturales, CSIC, José Gutiérrez Abascal, 2, 28006 Madrid, España. 2 Laboratorio de Arqueozoología “M. en C. Ticul Álvarez Solórzano”, Moneda 16, Col. Centro, 06060 México, D. F., Mexico. 3 Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, Mexico. * [email protected] ABSTRACT tral; y un nuevo caballo de pequeño tamaño Equus cedralensis sp. nov., conocido hasta ahora sólo en localidades mexicanas. El conocimiento A detailed study has been undertaken with an unique horse de la presencia conjunta de estas tres especies en el Pleistoceno tardío de bone deposit at Cedral, San Luis Potosí, central Mexico. Morphologi- México (género Equus sp.) es importante para entender los modelos de cal and morphometrical characters are used, as well as bivariate and diversidad y extinción en los primeros tiempos de la presencia humana multivariate statistics for both cranial and postcranial elements, and en el continente. -
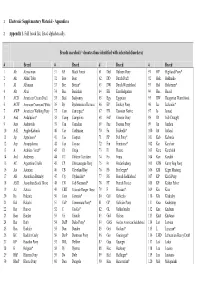
Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

Tulane Studies Tn Geology and Paleontology Pliocene
TULANE STUDIES TN GEOLOGY AND PALEONTOLOGY Volu me 22, Number 2 Sepl<'mber 20. l!J8~) PLIOCENE THREE-TOED HORSES FROM LOUISIANA. WITH COMMENTS ON THE CITRONELLE FORMATION EAHL M. MANNING MUSP.UM OF'GEOSCIF:NCE. LOUISJJ\NA STATE UNIVF:RSlTY. JJATO.\I ROI.JG/<. LOL'/S//\;\':1 and llRUCE J. MACFADDlrn DEJ>ARTM/<:NTOF NATUH/\LSCIENCES. F'LORJD/\ MUSf:UM Of<'NJ\TUIV\/, lllSTOUY UNIVERSITY OF FLOH!IJJ\. GJ\/NESVlU.E. Fl.OH/DA CONTENTS Page T. ABSTRACT 3.5 II INTRODUCTION :l5 Ill. ACKNOWLEDGMENTS :rn TV . ABBREVIATIONS :l7 V. SYSTEMATIC PALEONTOLOGY ;37 VI. AGE OF THE TUNICA HILLS HIPPARIONINES 38 VIL STRATIGRAPHIC PROVENIENCE 38 Vlll. PLIOCENE TERRESTRIAL VERTEBRATES OF THE GULF AND ATLANTIC COASTAL PLAIN .JO IX. COMMENTS ON THE CITRONELLE FORMATION .JI X. AGE OF THE CITRONELLE 42 XL TH E CITRONELLE FORMATION IN nm TUNICA HILLS .t:1 XII. LITERATURE CITED l.J January of 1985, the senior author was L ABSTRACT shown a large collection of late Pleistocene Teeth and metacarpals of early Pliocene (Rancholabrean land-mammal agel ver (latest Hemphillian land-mammal age) tebrate fossils from the Tunica Hills of three-toed (hipparionine) horses are de Louisiana (Fig. I) by Dr. A. Bradley scribed from the Tunica Hills of West McPherson of Centenary College, Feliciana Parish in east-central Louisiana. Shreveport. McPherson and Mr. Bill Lee An upper molar perta ins to Nannippus of Balon Rouge had collected fossils from minor, known from the Hcmphillian of that area since about 1981. Among the Central and North America, and two teeth standard assemblage of Rancholabrean and two distal metacarpals pertain to a re taxa (e.g. -

On the Validity of Equus Laurentius Hay, 1913 Eric Scott, Division of Geological Sciences, San Bernardino County Museum, Redlands, California Thomas W
On the Validity of Equus laurentius Hay, 1913 Eric Scott, Division of Geological Sciences, San Bernardino County Museum, Redlands, California Thomas W. Stafford, Jr., Stafford Research Laboratories, Boulder, Colorado Russell W. Graham, Department of Earth and Space Sciences, Denver Museum of Nature and Sciences, Denver, Colorado Larry D. Martin, Division of Vertebrate Paleontology, Natural History Museum and Biodiversity Research Center, University of Kansas, Lawrence, Kansas ABSTRACT BACKGROUND The species Equus laurentius Hay, 1913 has been controversial since its The species Equus laurentius was named from an associated skull and mandible recovered from a sandbar on the north side of the Kansas River near Lawrence in Douglas County, inception. Authorities have differed over the interpretation of this taxon; Kansas (Hay, 1913). The specimen (KUVP 347) was presumed to be of Pleistocene age, as it was found in apparent association with a femur assigned to Smilodon from the same some have considered it a legitimate Pleistocene horse species, while sandbar and appeared mineralized. E. laurentius was considered by Hay (1913) to be a horse similar in size to smaller domestic breeds, with rather small cheek teeth that exhibited others have proposed that the name is invalid on the basis that the relatively simple enamel infoldings or plications. Measurements provided by Hay (1913) for KUVP 347 did not distinguish the specimen from extant E. caballus (Hay, 1927). holotype specimen is a mineralized skull of a recent horse. As the taxon is still frequently employed in studies of Pleistocene equids, it is important Several subsequent authors (Matthew, 1926; Savage, 1951; Winans, 1985, 1989) considered the holotype of Equus laurentius to be a skull of a modern horse, Equus caballus to correctly assess its validity.