Quality Management in the Cardiac Catheterization Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bundled PCI Services in a Non-Hospital Cath Lab: Environmental Scan/Annotated Bibliography
Bundled PCI Services in a Non-Hospital Cath Lab: Environmental Scan/Annotated Bibliography The research questions guiding the environmental scan and the search strategy are described in detail in the attached appendix. The components of the annotated bibliography below (with links and citations to sources) are grouped into topic areas with main points relevant to the proposal review outlined below. BRIEF DESCRIPTION OF THE PROPOSAL The “Bundled PCI Services in a Non-Hospital Cath Lab” proposal aims to reduce costs of Percutaneous Coronary Interventions (PCIs) by giving Medicare patients the option to have the procedure performed in a non-hospital outpatient cardiac catheterization (cath) lab. Clearwater Cardiovascular Consultants, the submitting organization, proposes Bundled PCI services to allow qualified non-hospital cath labs the ability to perform PCIs, beginning with CCC’s own established cath lab as a limited scale test case to develop appropriate criteria, with expansion to at least two additional cath labs in the next year. SUBMITTING ORGANIZATION Clearwater Cardiovascular Consultants Clearwater Cardiovascular Consultants (CCC) is a cardiovascular medicine group founded in 1975, located in Clearwater, Florida, and comprised of 20+ physicians. Prior to 2016, CCC physicians provided PCI services at a hospital outpatient cardiac cath lab owned by Morton Plant Hospital (MPH), located near the emergency room/hospital outpatient lab. On January 1, 2016, CCC acquired this cath lab from MPH, retaining the same staff. http://www.ccicheart.com/ https://baycare.org/mph Bundled PCI Services in a Non-Hospital Cath Lab, June 2018 1 CURRENT ISSUES AND CONCERNS WITH MEDICARE PAYMENT FOR PCI CCC proposes an approach that will reduce spending for Anchor PCI procedures by an estimated $1,285 for a single vessel PCI and $3,105 for a multi-vessel PCI by performing PCIs in more cost-effective facilities as appropriate. -

Health Facilities and Services Review Board
STATE OF ILLINOIS HEALTH FACILITIES AND SERVICES REVIEW BOARD 525 WEST JEFFERSON ST. • SPRINGFIELD, ILLINOIS 62761 •(217) 782-3516 FAX: (217) 785-4111 DOCKET NO: BOARD MEETING: PROJECT NO: September 14, 2021 21-016 PROJECT COST: H-04 FACILITY NAME: CITY: Original: $170,520,604 NorthShore Glenbrook Hospital Glenview TYPE OF PROJECT: Substantive HSA: VII PROJECT DESCRIPTION: The Applicant [NorthShore University HealthSystem] is asking the State Board approve establishment of an open-heart surgery category of service, the addition of 8 cardiac cath labs, and the addition of 6 surgery rooms at Glenbrook Hospital in Glenview, Illinois. The cost of the project is $170,520,604. The expected completion date is December 31, 2024. The purpose of the Illinois Health Facilities Planning Act is to establish a procedure (1) which requires a person establishing, constructing or modifying a health care facility, as herein defined, to have the qualifications, background, character and financial resources to adequately provide a proper service for the community; (2) that promotes the orderly and economic development of health care facilities in the State of Illinois that avoids unnecessary duplication of such facilities; and (3) that promotes planning for and development of health care facilities needed for comprehensive health care especially in areas where the health planning process has identified unmet needs. Cost containment and support for safety net services must continue to be central tenets of the Certificate of Need process. (20 ILCS 3960/2) The Certificate of Need process required under this Act is designed to restrain rising health care costs by preventing unnecessary construction or modification of health care facilities. -

Curriculum in Interventional Cardiology
CURRICULUM IN INTERVENTIONAL CARDIOLOGY Educational Goals The educational goals of this program are to train fellows in a specialized cardiovascular disease area requiring technical, educational and research skills involved in interventional cardiology. The knowledge base for interventional cardiology has become increasingly well-defined as a result of unparalleled programs in basic and clinical research in atherosclerosis, coronary disease, cardiomyopathy and valvular heart disease. Our educational goals for training in interventional cardiology are aligned with and guided by the recommendations of the ACC/SCAI/ AHA task force on optimal adult interventional cardiology training programs as follows: • To understand the effectiveness and limitations of coronary interventional procedures in order to select patients and procedure types appropriately. • To achieve the appropriate cognitive knowledge and technical skills needed to perform interventional cardiac procedures at the level of quality attainable through the present state of the art. • To foster an attitude of life-long learning and critical thinking skills needed to gain from experience and incorporate new developments. • To understand and commit to quality assessment and improvement in procedure performance. All trainees must be skilled in obtaining a history and physical examination of the cardiovascular system, specifically as it relates to the performance of procedures, management of the patient during the procedure and also post-procedural follow-up. All trainees must be familiar with the role of aging and psychogenic factors in the production of symptoms, as well as emotional and physical response of the patient to cardiovascular disease. In addition, trainees must be familiar with specific pathophysiology as it relates to the development of cardiovascular disease particularly that of acute coronary syndromes and their management in the cardiac catheterization laboratory. -

Catheterization Lab Patient Instructions Catheterization Lab (520) 324-5034 Inside Front Cover Catheterization Lab Patient Instructions
Catheterization Lab Patient Instructions Catheterization Lab (520) 324-5034 Inside Front Cover Catheterization Lab Patient Instructions Catheterization Lab (520) 324.5034 5301 E. Grant Road, Tucson, AZ 85712 Table of Contents: Catheterization Lab Patient Instructions The day before your procedure ........................... 1 Medications ............................................ 1 What to bring with you to the hospital ....................... 1 When you arrive at the hospital .......................... 2 Preparing for your procedure .............................. 3 During your procedure .................................. 4 After your procedure .................................... 4 Before you are discharged ............................... 5 After your release ....................................... 5 Activity restrictions after heart catheterization. .6 Things to keep in mind .................................. 6 Cath Lab/Special Procedures Pre-Procedural Medication Instructions Diabetes medication ..................................... 7 Other medications .................................... 9 Catheterization Lab Patient Instructions The day before your procedure • Do not eat or drink anything 8 hours prior to procedure. However, it is okay to have a small sip of water with your medications. • You must have a responsible adult drive you home if you are having any type of sedation or anesthesia. • Take a shower either the night before, or the morning of your procedure. • Inform your doctor if you are unable to lie flat on your back for -
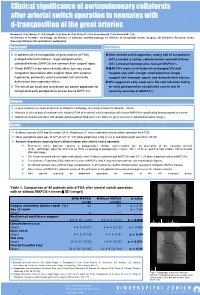
Clinical Significance of Aortopulmonary Collaterals After Arterial Switch Operation in Neonates with D-Transposition of the Great Arteries
Clinical significance of aortopulmonary collaterals after arterial switch operation in neonates with d-transposition of the great arteries Navarini S. (1,4), Balmer C. (1,4), Hug M. (2,4), Dave H. (3,4), Prêtre R. (3,4), Kretschmar O. (1,4), Knirsch W. (1,4) (1) Division of Pediatric Cardiology, (2) Division of Intensive Care/Neonatology, (3) Division of Congenital Cardiac Surgery, (4) Children’s Research Centre, University Children’s Hospital Zürich, Switzerland Background Conclusion In patients with d-transposition of great arteries (d-TGA), After arterial switch operation, nearly half of our patients enlarged bronchial arteries / major aortopulmonary (43%) needed a cardiac catheterisation and half of them collateral arteries (MAPCA) are common after surgical repair. (20%) showed hemodynamic relevant MAPCA’s. Those MAPCA’s are often clinically silent, but may cause 2 MAPCA’s were a risk factor for prolonged ICU and congestive heart failure after surgical repair with systemic hospital stay with a longer ventilation time, longer hypoxemia, pulmonary volume overload, left ventricular support with inotropic agents and delayed chest closure. dysfunction and respiratory failure. We suggest an early work-up in the cath lab when facing The aim of our study was to evaluate our patient population for an early postoperative complicated course due to complicated early postoperative course due to MAPCA’s. coronary anomaly or MAPCA’s. 3 Methods 4-year retrospective study at Division of Pediatric Cardiology, Universitiy Children’s Hospital , Zürich. Analysis of clinical data of neonates with simple d-TGA after arterial switch operation with known MAPCA’s complicating the postoperative course. -

Index of Microcirculatory Resistance: the Basics
Index of Microcirculatory Resistance: The Basics William F. Fearon, MD Associate Professor of Medicine Director, Interventional Cardiology Stanford University Medical Center Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company Grant/Research Support St. Jude Medical, Medtronic, NHLBI Consulting Fees/Honoraria Medtronic Major Stock Shareholder/Equity Royalty Income Ownership/Founder Intellectual Property Rights Other Financial Benefit Minor stock options: HeartFlow Assessment of the Microvasculature Diagnostic Challenge Epicardial CAD Lanza and Crea. Circulation 2010;121:2317-2325. Assessment of the Microvasculature Diagnostic Challenge Epicardial CAD Microvascular Dysfunction Lanza and Crea. Circulation 2010;121:2317-2325. Assessment of the Microvasculature Extremely challenging diagnosis Heterogeneous patient population Variety of pathogenetic mechanisms Poor anatomic resolution Potentially patchy nature of the disease Therefore, assessment of the microvasculature is primarily functional and not anatomic Evaluating the Microcirculation… …in the Cath Lab TIMI Myocardial Perfusion Grade: Evaluating the Microcirculation… …in the Cath Lab TIMI Myocardial Perfusion Grade: Easy to obtain Specific for microvasculature Predictive of outcomes in large studies Drawbacks: Qualitative Interobserver variability Not as useful in smaller studies Doppler Wire -

Mitral Valvuloplasty
MITRAL VALVULOPLASTY Appointment Date: __________________________ WHAT TIME SHOULD I ARRIVE? The Heart Lab Staff will call you between 2 – 4 p.m. the day prior to your test with your arrival time. The approximate time of your procedure is not known until the day before your test. If you will not be available at that time to receive the call, or if you don’t get a call by 4 p.m., please call to confirm your arrival time. Heart Cath Lab: 330-363-4230 Ambulatory Cardiac Unit: 330-363-5016 (if no answer in Heart Lab) Please use the Bedford (6th Street) entrance on arrival to the hospital. The visitor lot on 6th Street (lot 3) is the closest parking INSTRUCTIONS: Please review the written information given to you prior to coming to the hospital. You will be scheduled for a pretest appointment for your pre-admission testing. Do not eat or drink anything except for sips of water with your medication(s) after 12 midnight the night before your procedure. Please bring all your medications to the hospital the day of your procedure. They will be sent home with you. You will not be permitted to take medications brought in from home while you are in the hospital. If you are allergic to shellfish, iodine or X-ray dye, please tell the staff. Other instructions________________________________________________ Medication Instructions: Your doctor office or nurse coordinator will give you instructions about what medications to take (or not take) before your procedure. LOCATION: Ambulatory Cardiac Unit, located in Aultman Heart and Vascular Center. -

Unlock Ep Lab Capacity with Medtronic Cryoballoon Treat More Patients
UNLOCK EP LAB CAPACITY WITH MEDTRONIC CRYOBALLOON TREAT MORE PATIENTS. SAVE TIME. REDUCE STRESS. University Clinical Centre Rijeka, Croatia ABLATION AS A TREATMENT FOR AF The 2016 European Society of Cardiology (ESC) serious bleeding, or cardiac arrest, or on all-cause guidelines recommend PVI with catheter ablation, mortality. However, a per-protocol analysis censoring without further substrate modification, as first- the 9.2% of ablation patients who did not get line therapy to treat patients with AF. Catheter the procedure and the 27.5% of the drug group ablation can be performed with radiofrequency (RF) who crossed over to ablation shows substantial or cryothermal energy.5 An alternative to catheter advantages to the ablation procedure: 33% relative ablation is antiarrhythmic drug therapy. reduction in the primary composite endpoint, and Results from CABANA, a large multicentre, 40% relative reduction in all-cause mortality. Both randomised controlled trial comparing catheter analyses showed a significant 17% relative reduction ablation to drug therapy for the treatment of AF, in death or cardiovascular hospitalisation associated did not demonstrate a significant difference in the with catheter ablation.6 primary composite endpoint of disabling stroke, ATRIAL FIBRILLATION: CRYOBALLOON ABLATION THE CURRENT EPIDEMIC ECONOMIC VALUE Atrial fibrillation (AF) is the most common sustained FIRE AND ICE,10 the largest multicentre, prospective, operator and volume-dependent. Cryoballoon cardiac arrhythmia, affecting 1–2% of the general randomised trial that compared the efficacy and ablation results were more consistent regardless population around the world.1,2 In Europe, there are safety of Cryoballoon ablation and RF ablation, of centre or operator experience. -

19930005305.Pdf
FINAL REPORT Submitted to GE Government Services, Inc. Houston, Texas 77058 February 10, 1992 ADVANCED SENSORS TECHNOLOGY SURVEY Approvals: Arthur E. Schulze Vice-president Lovelace Scientific Resources, Inc. Daniel W. Barineau Job Order Task Manager GE Government Services David R. Proctor 2 Job Order Task Manager NASA/ JSC 012 ADVANCED SENSORS TECHNOLOGY SURVEY TEAM MEMBERS LOVELACE SCIENTIFIC RESOURCES, INC. - x, Project Manager: Arthur E. Schulze, P.E. Contributors: Tommy G. Cooper, P.E. CCS, Inc. David J. Costello Optex, Inc. Jerry G. Davis LSR, Inc. Richard L. Horst, Ph.D. Man-Made Systems Corp. Charles S. Lessard, Ph.D. Texas A&M University H. Herbert Peel Southwest Research Institute Robert Tolliver LSR, Inc. GE GOVERNMENT SERVICES Task Manager: Daniel W. Barineau NASA/JOHNSON SPACE CENTER Task Manager: David R. Proctor TABLE OF CONTENTS SECTION PAGE NO, TABLE OF CONTENTS . .1 EXECUTIVE SUMMARY . .1 ACRONYMS AND ABBREVIATIONS . 13 1.0 INTRODUCTION . 15 1.1 OBJECTIVES . 15 1.2 METHODOLOGY. 15 1.3 SCOPE . 16 2.0 WHAT IS ADVANCED SENSOR TECHNOLOGY? . 17 2.1 DEFINITIONS . 17 2.2 DESCRIPTIONS . 19 2.3 TWO "SMART' SENSOR EXAMPLES . 21 3.0 NASA PROGRAM APPLICATIONS . 25 3.1 BACKGROUND . 25 3.2 "SMART' SENSOR USAGE IN THE BMAC PROGRAM . 26 3.2.1 Smart Electrophysiological Sensors . 26 3.2.2 Smart Physical Transducer Sensors . 27 3.2.3. Smart Chemically Active Sensors . 29 3.3 WIRELESS SENSOR AND CONTROL PACKAGES . 35 3.4 SYSTEMS INTEGRATION ISSUES . 36 3.4.1 Medical Information Bus . 39 3.4.2 Smart Sensor/MIB/SSF Relationships . 44 3.5 HUMANFACTORSISSUES . -

2014 Imaging in and Around the Cath
Imaging in and Around the Cath Lab Thomas Smith, MD FACC University of California, Davis Disclosures • I have no disclosures pernent to this presentaon. Modalies in Procedure Lab • Intracardiac Echocardiography (ICE) • Transthoracic Echocardiography (TTE) • Transesophageal Echocardiography (TEE) Key Points to Ancipate Imaging • Which chamber or valve? • General anesthesia? Primary Procedures Requiring Imaging • Exclusion of LAA thrombus – Afib/fluer ablaon – Mitral valvuloplasty • Transseptal – most procedures involving LA or LV • Valve replacement – AV, ?MV, ? PV • Valvuloplasty – Mitral or aorc • Valve repair – MitraClip – Paravalvular leak repair • Pericardiocentesis • Alcohol septal ablaon • PFO or ASD closure Transseptal Procedures – Mitral Valvuloplasty – EP Ablaons – Paravalvular Leak – Le Atrial Appendage Closure – Mitral Repair with MitraClip system SP LA Length ED H SS RA Bicaval TEE IVC SVC EV Caval flow into the RA LA IVC SVC EV RA Intracardiac Echocardiography Intracardiac Echocardiography Intracardiac echo Transesophageal Echocardiography Esophagus LAA LA Appendage – Thrombus Mitral valve repair MITRACLIP MitraClip Procedure Animaon Imaging Objecves in MitraClip • Exclude LAA thrombus. • Document preprocedure MR. • Guide crossing point for trans-septal • Guide catheters in the LA to appropriate posion. • Guide clip posioning. • Assess for safe capture of leaflets. • Post-procedure MR assessment. • Assess for complicaons. Endovascular Mitral Repair System 3D Transesophageal Echo SVC Ao Ao * * FO AV View from RA VieW from LA Transeptal -
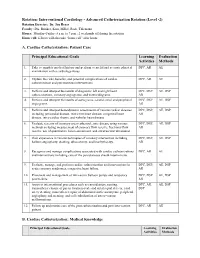
Interventional Cardiology - Advanced Catheterization Rotation (Level -2) Rotation Director: Dr
Rotation: Interventional Cardiology - Advanced Catheterization Rotation (Level -2) Rotation Director: Dr. Jon Resar Faculty: Drs. Brinker, Kass, Miller, Rade, Thiemann, Hours: Monday–Friday: 8 a.m. to 7 p.m.; 2 weekends off during the rotation. Home call: fellows will also take “home call” after hours A. Cardiac Catheterization: Patient Care Principal Educational Goals Learning Evaluation Activities Methods 1. Take a complete medical history and perform a careful and accurate physical DPC, AR AE examination with a cardiology focus. 2. Explain the risks, benefits, and potential complications of cardiac DPC, AR AE catheterization and percutaneous interventions. 3. Perform and interpret the results of diagnostic left and right heart DPC, DSP, AE, DSP catheterizations, coronary angiograms, and ventriculograms. AR 4. Perform and interpret the results of aortograms, carotid, renal and peripheral DPC, DSP, AE, DSP angiograms. AR 5. Perform and interpret hemodynamic assessments of various cardiac diseases DPC, DSP, AE, DSP including pericardial disease, restrictive heart disease, congenital heart AR disease, intra-cardiac shunts, and valvular heart disease. 6. Evaluate severity of coronary artery atherosclerotic disease using various DPC, DSP, AE, DSP methods including measurement of coronary flow reserve, fractional flow AR reserve, use of quantitative lesion assessment, and intravascular ultrasound. 7. Gain experience in various techniques of coronary intervention including DPC, DSP, AE, DSP balloon angioplasty, stenting, atherectomy, and brachytherapy. AR 8. Recognize and manage complications associated with cardiac catheterizations DPC, AR AE and interventions including care of the percutaneous sheath insertion site. 9. Evaluate, manage, and perform cardiac catheterization and interventions in DPC, DSP, AE, DSP acute coronary syndromes, congestive heart failure. -

Patient Teaching Guide
Patient Teaching Guide Percutaneous Intervention for Coronary Artery and Peripheral Vascular Diseases Table of Contents Overview The Procedure 2 . .Your Heart and Circulatory System 13 . .Preparing for Your Procedure Cardiovascular Diseases 14 . .Before the Procedure 3 . .Coronary Artery Disease (CAD) 15 . .The Catheterization Laboratory 4 ..... .CAD: Risk Factors 15 . .Inserting the Guiding Catheter 5 ..... .CAD: The Diagnosis 15 . .Injecting the Dye 6 ..... .CAD: Treatment Options 16 . .Angioplasty 17 . .Stent Implantation 8 . .Peripheral Vascular Disease (PVD) 18 . .Atherectomy 9 ..... .PVD: Risk Factors 19 . .After the Procedure 10 ..... .PVD: The Diagnosis Recovery 11 ..... .PVD: Treatment Options 22 . .Your Recovery 23 . .Lifestyle Changes Glossary 24 . .Glossary Overview Your Heart and Circulatory System The heart is a muscle that pumps blood to all parts of the body. The blood carries oxygen and nutrients that your body needs to work correctly. Blood flows out from the heart through the aorta, which is the largest artery in the body. All of the major arteries branch from the aorta and carry blood to all parts of the body. Blood vessels called “veins” return the blood to the heart. After picking up oxygen from the lungs, the blood is pumped out to the body again. The movement of blood through the heart, lungs and body is called “circulation.” As the heart pumps, it produces a pulse. Counting the pulse tells us how many times the heart pumps or beats per minute. For the heart to be able to function properly, it needs a constant supply of oxygen-filled blood. The vessels that supply this blood to the heart are called coronary arteries.