July 2011 Entire Research, Vol.-3, Issue-III 1 Inhibition Efficiency of N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Risky Sex, Addictions, and Communicable Diseases in India: Implications for Health, Development, and Security
Risky Sex, Addictions, and Communicable Diseases in India: Implications for Health, Development, and Security Rajan Gupta Theoretical Division Los Alamos National laboratory, Los Alamos, NM 87545 [email protected] LAUR 02-5305 This monograph was published as Special Report 8 in the Health and Security Series by the Chemical and Biological Arms Control Institute (CBACI), Washington D.C., September 2004. ABSTRACT: This monograph provides a comprehensive and unifying view of a number of health issues confronting India and how, over time, they could impact the stability and security of the nation. New pandemics like HIV/AIDS have confounded attempts at containment because their spread highlights vulnerabilities in social and political norms and behaviors that have historically been ignored. Their spread also exposes a highly inadequate medical and educational infrastructure. To stop the spread of communicable diseases for which risky individual lifestyles and behaviors, societal norms and beliefs, poverty and lack of empowerment, and stigma and discrimination are major factors it is necessary to examine the system as a whole and to develop new paradigms and tools. Sexually transmitted infections and addictions to alcohol and drugs have emerged as a major interconnected global threat. This monograph makes the case that India is highly vulnerable to this threat and major policy changes, an unprecedented cooperation between public and private sector, and an order of magnitude more investment in health and education is needed to prevent a runaway -
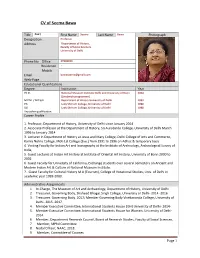
CV of Seema Bawa
CV of Seema Bawa Title Prof./ First Name Seema Last Name Bawa Photograph Designation Professor Address Department of History, Faculty of Social Sciences University of Delhi Phone No Office 27666659 Residence - Mobile Email [email protected] Web-Page Educational Qualifications Degree Institution Year Ph.D. National Museum Institute Delhi and University of Bonn 2004 (Sandwich programme) M.Phil. / M.Tech. Department of History University of Delhi 1993 PG Lady Shriram College, University of Delhi 1990 UG Lady Shriram College, University of Delhi 1988 Any other qualification Career Profile 1. Professor, Department of History, University of Delhi since January 2014 2. Associate Professor at the Department of History, Sri Aurobindo College, University of Delhi March 1996 to January 2014 3. Lecturer in Department of History at Jesus and Mary College, Delhi College of Arts and Commerce, Kamla Nehru College, Moti Lal College (Eve.) from 1991 to 1996 on Adhoc & temporary basis 4. Visiting Faculty for Indian Art and Iconography at the Institute of Archeology, Archeological Survey of India, 5. Guest Lectures at Indian Art History at Institute of Oriental Art History, University of Bonn 2000 to 2002 6. Guest Faculty for University of California, Exchange students over several semesters on Ancient and Modern Indian Art & Culture at National Museum Institute. 7.. Guest Faculty for Cultural History M.A (Tourism), College of Vocational Studies, Univ. of Delhi in academic year 1999-2000. Administrative Assignments 1. In-Charge, The Museum of Art and Archaeology, Department of History, University of Delhi 2. Treasurer, Governing Body, Shaheed Bhagat Singh College, University of Delhi- 2014 -2016 3. -

Current Awareness Bulletin
No.9-10 September-October 2018 Current Awareness Bulletin Centre for Women’s Development Studies (An autonomous research institute supported by the Indian Council of Social Science Research) 25, Bhai Vir Singh Marg (Gole Market), New Delhi-110001, India. Ph.: 011-9555231324; 9555231354. Fax: 91-23346044 1 E-mail: [email protected] | URL: http://www.cwds.ac.in/library/ CONTENTS Section 1: Journals/ Periodicals/ Newsletters Articles Ageing ............................................................................ 001-002 Child Abuse .................................................................... 003-005 Child Labour................................................................... 006 Children .......................................................................... 007-020 Employment ................................................................... 021 Family ............................................................................ 022-023 Family Planning .............................................................. 024 Feminism ........................................................................ 025-028 Gender Studies................................................................ 029 Girl Child........................................................................ 030-037 Health ............................................................................. 038 Laws ............................................................................... 039-051 Marriage ........................................................................ -

Trade Marks Journal No: 1816 , 25/09/2017 Class 35
Trade Marks Journal No: 1816 , 25/09/2017 Class 35 Advertised before Acceptance under section 20(1) Proviso 1447319 28/04/2006 UNMESH AMARCHAND KANTAWALA. KAUSHABH UNMESH KANTAWALA trading as ;M/S. KANTAWALA DESIGNER JEWELLERS. SWADESHI MILLS ESTATE, 1ST FL., TATA RD. NO. 1, OPERA HOUSE, MUMBAI - 400 004. MANUFACTURERS & MERCHANTS. REGD. UNDER THE INDIAN PARTNERSHIP ACT. Address for service in India/Agents address: M. P. MIRCHANDANI & CO. RAM MANSION, IST FLOOR, ROOM NO.4, N.F. ROAD, NEAR CITY WALK, COLABA, MUMBAI - 400 001. Proposed to be Used MUMBAI RETAIL OUTLETS AND DEPARTMENTAL STORES. REGISTRATION OF THIS TRADE MARK SHALL GIVE NO RIGHT TO THE EXCLUSIVE USE OF THE WORD "JEWELS". 4993 Trade Marks Journal No: 1816 , 25/09/2017 Class 35 Advertised before Acceptance under section 20(1) Proviso 1610229 10/10/2007 JATIN VARMA B-9/1C, Vasant Vihar, NewDelhi-110 053. Manufacturers & merchants (Proprietor) (An Indian National) Address for service in India/Agents address: THE ACME COMPANY B-41, NIZAMUDDIN EAST, NEW DELHI - 110013. Used Since :01/04/2007 DELHI ADVERTISING, PUBLICITY AND BUSINESS SERVICES, INTERNET, COMMERCIAL ADMINISTRATION, RENTAL OF OFFICE MACHINES AND EQUIPMENT, DEMONSTRATION OF GOODS, PRODUCTION OF SOUND/VIDEO RECORDING FOR ADVERTISEMENT, PUBLICITY AND MARKETING PURPOSES ALL INCLUDED IN CLASS 35. 4994 Trade Marks Journal No: 1816 , 25/09/2017 Class 35 INDIA VALUE FUND Advertised before Acceptance under section 20(1) Proviso 1655490 19/02/2008 IVF ADVISORS PRIVATE LIMITED SUITE F9C, GRAND HYATT PLAZA, SANTACRUZ, MUMBAI - 400 055. MANUFACTURERS AND MERCHANTS Address for service in India/Agents address: DSK LEGAL. 4TH FLOOR, EXPRESS TOWERS, NARIMAN POINT, MUMBAI-400 021. -

Rate the Debate – Research Report
TELEVISION NEWS IN INDIA 31 DEC 2020 RATE THE DEBATE 1 Contents Introduction ............................................................................................................... 3 Today’s Context .......................................................................................................... 3 The Distorted Media Lens ........................................................................................... 4 Rate the Debate .......................................................................................................... 6 Methodology .............................................................................................................. 6 Appendix A –Anchor Analysis ..................................................................................... 9 Appendix B – Media Trial Analysis ............................................................................ 16 Appendix C – Rate the Debate Team ......................................................................... 24 2 Introduction In a rare conversation in 2012, BCCL Managing Director Vineet Jain told The New Yorker the philosophy underpinning the transformation of the newspaper The Times of India – “We are not in the newspaper business, we are in the advertising business.” There-casting means that readership became the product: deliver a newspaper that attracts subscriptions, and you can sell the readership to advertisers in a variety of ways. It cemented what The New Yorker described as changes in the newspaper initiated by then Vice Chairman -

Roopa Pai the Author of the Gita for Children on Mythology, Philosophy and Wisdom for Grownups
November 2019 Vol 3 Issue 10 `150 Roopa Pai The author of The Gita for Children on mythology, philosophy and wisdom for grownups FIT AND FAB @ 50 WARRIOR TALE BIKER DIARY 4 awesome 50-plus Dancer-filmmaker Swati 4 intrepid women divas share their beauty Bhise puts the spotlight set off on a daring and fitness secrets on the Rani of Jhansi ride across borders 2 | PERSONAL GROWTH L U X U R I O U S L Y H A N D C R A F T E D S K I N C A R E Handmade Soaps | Serums | Bath Salts | Face Masks and more TO ORDER, VISIT: FACEBOOK.COM/SOAPERYBYSIMRAN INSTAGRAM.COM/SIMRANGUJRALGAMBHIR S O A P E R Y B Y S I M R A N @ G M A I L . C O M NOVEMBER 2019 contents Doctor Art 06 Two doctors with a highly creative side All for Girls 12 New enterprises out to empower women Books of Wisdom 20 Cover personality and author Roopa Pai Fit and Fab @ 50 25 Four awesome divas share fitness secrets L U X U R I O U S L Y H A N D C R A F T E D S K I N C A R E The Warrior Queen’s Tale 34 Dancer and feminist filmmaker Swati Bhise Unfair Burden 38 A question about teachers’ maternity leave Bloom Where You’re Planted 40 Inspiring tales of midlife personal growth Handmade Soaps | Serums | Bath Salts | Face Masks and more In a Soup 52 Four healthy soup recipes for winter TO ORDER, VISIT: FACEBOOK.COM/SOAPERYBYSIMRAN 16 Fashion with Heart INSTAGRAM.COM/SIMRANGUJRALGAMBHIR Fashion designer and body- Bikers Without Borders positivity activist Kaveri Lalchand 62 Four women undertake an intrepid journey S O A P E R Y B Y S I M R A N @ G M A I L . -

Violence in Gujarat
April 2002 Vol. 14, No. 3(C) “WE HAVE NO ORDERS TO SAVE YOU” State Participation and Complicity in Communal Violence in Gujarat TABLE OF CONTENTS I. SUMMARY................................................................................................................................................4 II. RECOMMENDATIONS.....................................................................................................................9 To the State Government of Gujarat:............................................................................................................9 To the Government of India:......................................................................................................................10 To India’s Donors and Trading Partners: ....................................................................................................11 To International Lending Institutions:.........................................................................................................11 To International Humanitarian Organizations:.............................................................................................11 To United Nations Agencies:.....................................................................................................................11 III. MASSACRES IN GODHRA AND AHMEDABAD .................................................................................13 Godhra.....................................................................................................................................................13 -

Cwe-Clerks-Vii) Verification & Submission of Documents
A COMMON RECRUITMENT PROCESS FOR RECRUITMENT OF CLERKS (CWE-CLERKS-VII) VERIFICATION & SUBMISSION OF DOCUMENTS Reference is invited to provisional allotment of candidates for the post of Clerk at Indian Bank. The candidates are advised to report at the venue (Reporting Location) as mentioned in Table I on 09.07.2018 for Document Verification. Candidates are advised to bring originals of all the requisite documents mentioned in the Provisional Offer of Appointment. Only those candidates who are found eligible in the document verification process and have proficiency in the Official Language of the State/UT will be inducted and issued Posting Orders. After completion of the Induction process, all the eligible candidates will undergo Induction Training in batches. Batches I & II will undergo Induction Training from July 10-21st, 2018 at the location mentioned in Table I. Batches III & IV will join at the branches on 10.07.2018 and Induction Training for these candidates will be held from July 23-Aug 4, 2018 at Staff Training Centres (STC). The addresses of the Reporting Location & Induction Location are furnished in Table II. Candidates are advised to make necessary arrangement well in advance and report at the venue mentioned against their name for Document verification on 09.07.2018. Any request for change of venue, date & time of verification & submission of documents and venue of induction training / branch will not be entertained. Assistant General Manager (HRM) 25/06/2018 Disclaimer: Though utmost care has been taken while preparing the Roll Numbers of candidates based on the details provided by IBPS, the Bank reserves the right to rectify inadvertent errors, if any. -

Faculty Details
FACULTY DETAILS Title Prof./ First Name Seema Last Name Bawa Photograph Designation Professor Address A-1/65 Panchsheel Enclave New Delhi 110017 Phone No Office 27666659 Residence 26491265 Mobile Email [email protected] Web-Page Educational Qualifications Degree Institution Year Ph.D. National Museum Institute Delhi and University of Bonn 2004 (Sandwich programme) M.Phil. / M.Tech. Department of History University of Delhi 1993 PG Lady Shriram College, University of Delhi 1990 UG Lady Shriram College, University of Delhi 1988 Any other qualification Career Profile 1. Professor, Department of History, University of Delhi since January 2014 2. Associate Professor at the Department of History, Sri Aurobindo College, University of Delhi March 1996 to January 2014 3. Lecturer in Department of History at Jesus and Mary College, Delhi College of Arts and Commerce, Kamla Nehru College, Moti Lal College (Eve.) from 1991 to 1996 on Adhoc & temporary basis 4. Visiting Faculty for Indian Art and Iconography at the Institute of Archeology, Archeological Survey of India, 5. Guest Lectures at Indian Art History at Institute of Oriental Art History, University of Bonn 2000 to 2002 6. Guest Faculty for University of California, Exchange students over several semesters on Ancient and Modern Indian Art & Culture at National Museum Institute. 7.. Guest Faculty for Cultural History M.A (Tourism), College of Vocational Studies, Univ. of Delhi in academic year 1999-2000. Administrative Assignments 1. In-Charge, The Museum of Art and Archaeology, Department of History, University of Delhi 2. Treasurer, Governing Body, Shaheed Bhagat Singh College, University of Delhi- 2014 -2016 3. Treasurer. Governing Body. -

Library Collection Having Record ID Between '1' and '4618' Title Author Callnumber Accessionn O
Library Collection having Record ID Between '1' And '4618' Title Author CallNumber AccessionN o India 60 Towards a new paradigm Pandey , Ira , ed. , 954.08,PAN-I 4857 Deportation of Lala Lajpat Rai and Sardar Ajit Singh Singh , Ganda , ed. , 954.03508,SIN-D 886 Vol 4 Mudao moonchh lagao poonchh Patoria , Rajendra, H 891.437,PAT-M 2778 Bharteeya arthtantra etihas aur sanskriti Sen , Amartya , H 338.954,SEN-B 4371 House buliding and advance rules 1972 , 932 II My feudal lord Durrani, Tehmina, 920.02,DUR-M 2427 The code of civil procedure , 1908 India , Court 347.54,IND-C 3449 Amendments. 347.54,IND-C ; 1 3450 Jawaharlal Nehru An autobiography Jawaharlal Nehru 920.71 (N),JAW-J 446 memorial Fund . Bharatiya samajik sansthaye Natadi , Prakash H 306.81,NAT-B 4392 Narayan , The wakf amendment act , 1984 , 571 GP Multicultural citizenship A liberal theory of minority Kymlicka, Will , 323.1,KYM-M 3320 rights Princes Sasson , Jean , 823,SAS-P 5056 Human rights A critique Batra , T S , 341.48,BAT-H 214 Printed On : 22/08/2013 1 Library Collection having Record ID Between '1' And '4618' Title Author CallNumber AccessionN o From the Raj to Rajiv 40Years of Indian Tully , Mark , 320.954,TUL-F 2283 independence Social inequality and political structure Neelsen , John P , ed. 320.011,NEE-S 1321 , Bojh Dube , Bala , H 891.433,DUB-B 2813 Income tax act 1992 , 917 II Oxford advanced learner`s dictionary of current Oxford University R 423,OXF-L 2476 english Press . Islamic & comparative law quarterly Vol VII Mahmmod , Tahir , 340.59,MAH-I.1987 2916 Social sciences and social realities Indian Institute of 300.954,IND-S 542 Advanced Study . -

Current Awareness Bulletin
No.9-10 September-October 2019 Current Awareness Bulletin Centre for Women’s Development Studies (An autonomous research institute supported by the Indian Council of Social Science Research) 25, Bhai Vir Singh Marg (Gole Market), New Delhi-110001, India. Ph.: 011-9555231324; 9555231354. Fax: 91-23346044 E-mail: [email protected] | URL: http://www.cwds.ac.in/library/ 1 CONTENTS Section 1: Journals/ Periodicals/ Newsletters Articles Ageing ..............................................................................001-006 Child Abuse ......................................................................007-008 Child Labour .....................................................................009-010 Children ............................................................................011-026 Children’s Rights ..............................................................027 Demography .....................................................................028-031 Disabilities ........................................................................032-034 Education ..........................................................................035-037 Employment .....................................................................038 Feminism ..........................................................................039-042 Gender Studies ..................................................................043 Girl Child ..........................................................................044-052 Health ...............................................................................053-054 -

East Coast Railway Central Library
EAST COAST RAILWAY CENTRAL LIBRARY STOCK REPORT AS ON DATE 28-Aug-2018 ISBN No. Book Name Category Publication Author Rack/Shelf Price Stock In Hand 9788130403151 WORLDS GREAT SELECTED CHILDREN BOOKS PRINTLINE BOOKS PRINTLINE Rack-35 E 295 1 SHORT STORIES 9788130402864 BEST OF JOHN STORY BOOKS PRINTLINE BOOKS JOHN GLASSWORTHY Rack-19 B 295 1 GLASSWORTHY 9788130402802 BEST OF HARMAN MELVELLE STORY BOOKS PRINTLINE BOOKS HARAMAN MELVELLE Rack-19 B 295 1 9788130402857 BEST OF JOHN BHUCHAN STORY BOOKS PRINTLINE BOOKS JOHN BUCHAN Rack-19 B 295 1 9788130403007 BEST OF SIR ARTHUR CONAN STORY BOOKS PRINTLINE BOOKS SIR ARTHUR CONAN DOYLE Rack-19 B 295 1 DOYLE 9788130402970 BEST OF ROBERT LOUIS STORY BOOKS PRINTLINE BOOKS F ROBERT LOUIS Rack-19 B 295 1 STEVENSON STEVENSON 9788130402949 BEST OF MARK TWAIN STORY BOOKS PRINTLINE BOOKS MARK TWAIN Rack-19 B 295 1 9788130402840 BEST OF JANE AUSTEN STORY BOOKS PRINTLINE BOOKS JANE AUSTEN Rack-19 B 295 1 9788130402635 BEST OFDANIEL DEFOE STORY BOOKS PRINTLINE BOOKS DANIEL DEFOE Rack-19 B 295 1 9788130402512 BEST OF ALEXANDER DUMAS STORY BOOKS PRINTLINE BOOKS ALEXANDER DUMAS Rack-19 B 295 1 9788130402529 BEST OF ANNE BRONTE STORY BOOKS PRINTLINE BOOKS ANNE BRONTE Rack-19 B 295 1 9788130403083 BEST OF THE TRAGEDYKING STORY BOOKS PRINTLINE BOOKS WILLIAM SHAKESPERE Rack-19 B 295 1 WILLIAM SHAKESPERE 9788130402734 BEST OF GEORGE ELIOT STORY BOOKS PRINTLINE BOOKS GEORGE ELIOT Rack-19 D 295 1 9788130402628 BEST OF D.H. LAWRENCE STORY BOOKS PRINTLINE BOOKS D.H. LAWRENCE Rack-19 D 295 1 9788130402611 BEST OF F.