ASCCP Risk-Based Management Consensus Guidelines For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hysteroscopy with Dilation and Curretage (D & C)
501 19th Street, Trustees Tower FORT SANDERS WOMEN’S SPECIALISTS 1924 Pinnacle Point Way Suite 401, Knoxville Tn 37916 P# 865-331-1122 F# 865-331-1976 Suite 200, Knoxville Tn 37922 Dr. Curtis Elam, M.D., FACOG, AIMIS, Dr. David Owen, M.D., FACOG, Dr. Brooke Foulk, M.D., FACOG Dr. Dean Turner M.D., FACOG, ASCCP, Dr. F. Robert McKeown III, M.D., FACOG, AIMIS, Dr. Steven Pierce M.D., Dr. G. Walton Smith, M.D., FACOG, Dr. Susan Robertson, M.D., FACOG HYSTEROSCOPY WITH DILATION AND CURRETAGE (D & C) Please read and sign the following consent form when you feel that you completely understand the surgical procedure that is to be performed and after you have asked all of your questions. If you have any further questions or concerns, please contact our office prior to your procedure so that we may clarify any pertinent issues. Definition: HysterosCopy is an outpatient procedure that allows your doctor direct visualization of the inside of the uterine cavity (womb) by inserting a thin lighted telescope (hysteroscope) through the vagina (birth canal) and cervix, without making an abdominal incision. This procedure enables your doctor to examine the lining of the uterus, look for polyps, fibroids, scar tissue, blockages of the fallopian tubes, and abnormal partitions. In addition, this procedure allows your doctor to remove or surgically treat many of the abnormalities seen. Dilation and Curettage (D&C) allows your doctor to take a sample of the tissue that lines your uterus (endometrium) and/or to remove polyps, fibroid tumors, or hyperplasia. Suction D&C is used in cases of miscarriage. -

A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues
NORTH CAROLINA JOURNAL OF INTERNATIONAL LAW Volume 38 Number 2 Article 6 Winter 2013 A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues Kathleen Bradshaw Follow this and additional works at: https://scholarship.law.unc.edu/ncilj Recommended Citation Kathleen Bradshaw, A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues, 38 N.C. J. INT'L L. 601 (2012). Available at: https://scholarship.law.unc.edu/ncilj/vol38/iss2/6 This Note is brought to you for free and open access by Carolina Law Scholarship Repository. It has been accepted for inclusion in North Carolina Journal of International Law by an authorized editor of Carolina Law Scholarship Repository. For more information, please contact [email protected]. A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues Cover Page Footnote International Law; Commercial Law; Law This note is available in North Carolina Journal of International Law: https://scholarship.law.unc.edu/ncilj/vol38/iss2/ 6 A Discursive Approach to Female Circumcision: Why the United Nations Should Drop the One-Sided Conversation in Favor of the Vagina Dialogues KATHLEEN BRADSHAWt I. Introduction ........................................602 II. Background................................ 608 A. Female Circumcision ...................... 608 B. International Legal Response....................610 III. Discussion......................... ........ 613 A. Foreign Domestic Legislation............. ... .......... 616 B. Enforcement.. ...................... ...... 617 C. Cultural Insensitivity: Bad for Development..............620 1. Human Rights, Culture, and Development: The United Nations ................... ............... 621 2. -

A Study on Cervical Screening by PAP Smear and Correlation with Microbiological and Clinical Finding
International Journal of Health and Clinical Research, 2021;4(5):280-283 e-ISSN: 2590-3241, p-ISSN: 2590-325X ____________________________________________________________________________________________________________________________________________ Original Research Article A study on cervical screening by PAP smear and correlation with microbiological and clinical finding Sona Goyal1, Manish Kumar Singhal2*,Kamlesh Yadav3, Rachna Agrawal4, Neil Sharma 5 1Consultant Pathologist, Department of Pathology, NIA , Jaipur, Rajasthan, India 2Associate Professor, Department of Pathology, Govt Medical college, Bhartapur, Rajasthan, India 3Professor, Department of Pathology, SMS Medical college Jaipur, Rajasthan, India 4Assistant Professor, Department of Anatomy, Govt Medical college , Bhartapur, Rajasthan, India 5Assistant Professor, Department of Pathology, Govt Medical college, Bhartapur, Rajasthan, India Received: 03-01-2021 / Revised: 08-02-2021 / Accepted: 25-02-2021 Abstract Introduction: Cervical cancer is one of the most common cause in India with over 75% of incidence and mortality. The objective of cervical cancer screening, therefore, is the detection of these lesions before developing into invasive cervical cancer.Methods: This prospective study was carried out over 2 year at the Department of Obstetrics and Gynaecology in National Institute of Ayurveda, Jaipur. Pap smears were collected from 400 sexually active women who were more than 21 years of age.Result: Most common findings were Inflammatory lesion (46.5%), followed by NILM(30%). Atrophic smear was seen in 16 cases (4%), rest had abnormal cellular changes in the form of ASCUS (1.25 %), LSIL (2 %) and Carcinoma (1%).Conclusion : Inflammatory smear is most common cytological finding in premenopausal age group . Epithelial cell abnormality is most common finding in premenopausal and postmenopausal age groups. Pap smear examination can be coupled with culture and sensitivity of vaginal swab to provide adequate treatment. -

Developmental Abnormalities Easy to Misdiagnose
8 Gynecology O B .GYN. NEWS • March 15, 2005 Developmental Abnormalities Easy to Misdiagnose BY JANE SALODOF MACNEIL inappropriate surgery, according to Dr. even an entity called obstructed hemi- Abnormalities of the vulva include con- Contributing Writer Zurawin, chief of the section of pediatric vagina.” genital labial fusion, which he said could and adolescent gynecology at Baylor and Clitoral hypertrophy is the only devel- be corrected with a simple flap proce- H OUSTON — Developmental abnor- of the gynecology service at Texas Chil- opmental abnormality of the clitoris, ac- dure. Surgery is rarely used, however, for malities of the vulva and vagina are often dren’s Hospital in Houston. cording to Dr. Zurawin. It used to be acquired labial agglutination. “This is one easy to correct, but also easy to misdiag- “You need to be familiar with the syn- treated by clitoridectomy with “very un- of most common referrals from pediatri- nose, Robert K. Zurawin, M.D., warned at dromes before you treat. Many people satisfactory results,” he said, describing cians, because they don’t know what to do a conference on vulvovaginal diseases are confronted with these conditions, and more conservative procedures in use to- with it and they are afraid,” Dr. Zurawin sponsored by Baylor College of Medicine. they don’t know what they really are,” he day. “This is mainly a cosmetic problem said. Many physicians have not been trained said. “With the obstructions, for example, for patients, and the surgical management He attributed most cases to diaper rash, to recognize these rare disorders and, as a they may just think it is an imperforate hy- is resection of the enlarged clitoris,” he bubble baths, and detergents that can in- result, run the risk of doing excessive or men and are not even aware that there is said. -

Vaginal Screening After Hysterectomy in Australia
CATEGORY: BEST PRACTICE Vaginal screening after hysterectomy in Australia Objectives: To provide advice on vaginal This statement has been developed and screening after hysterectomy. reviewed by the Women’s Health Committee and approved by the RANZCOG Target audience: Health professionals Board and Council. providing gynaecological care. A list of Women’s Health Committee Values: The evidence was reviewed by the Members can be found in Appendix A. Women’s Health Committee (RANZCOG), and applied to local factors relating to Disclosure statements have been received Australia. from all members of this committee. Background: This statement was first developed by Women’s Health Disclaimer This information is intended to Committee in November 2010 and provide general advice to practitioners. This reviewed in March 2020. information should not be relied on as a substitute for proper assessment with respect Funding: This statement was developed by to the particular circumstances of each RANZCOG and there are no relevant case and the needs of any patient. This financial disclosures. document reflects emerging clinical and scientific advances as of the date issued and is subject to change. The document has been prepared having regard to general circumstances. First endorsed by RANZCOG: November 2010 Current: March 2020 Review due: March 2023 1 1. Introduction In December 2017, the National Cervical Screening Program in Australia changed from 2 yearly cervical cytology testing to 5 yearly primary HPV screening with reflex liquid-based cytology for those women in whom oncogenic HPV is detected in women aged 25–74 years. New Zealand has not yet transitioned to primary HPV screening. -

2021 – the Following CPT Codes Are Approved for Billing Through Women’S Way
WHAT’S COVERED – 2021 Women’s Way CPT Code Medicare Part B Rate List Effective January 1, 2021 For questions, call the Women’s Way State Office 800-280-5512 or 701-328-2389 • CPT codes that are specifically not covered are 77061, 77062 and 87623 • Reimbursement for treatment services is not allowed. (See note on page 8). • CPT code 99201 has been removed from What’s Covered List • New CPT codes are in bold font. 2021 – The following CPT codes are approved for billing through Women’s Way. Description of Services CPT $ Rate Office Visits New patient; medically appropriate history/exam; straightforward decision making; 15-29 minutes 99202 72.19 New patient; medically appropriate history/exam; low level decision making; 30-44 minutes 99203 110.77 New patient; medically appropriate history/exam; moderate level decision making; 45-59 minutes 99204 165.36 New patient; medically appropriate history/exam; high level decision making; 60-74 minutes. 99205 218.21 Established patient; evaluation and management, may not require presence of physician; 99211 22.83 presenting problems are minimal Established patient; medically appropriate history/exam, straightforward decision making; 10-19 99212 55.88 minutes Established patient; medically appropriate history/exam, low level decision making; 20-29 minutes 99213 90.48 Established patient; medically appropriate history/exam, moderate level decision making; 30-39 99214 128.42 minutes Established patient; comprehensive history exam, high complex decision making; 40-54 minutes 99215 128.42 Initial comprehensive -

FGM in Canada
Compiled by Patricia Huston MD, MPH Scientific Communications International, Inc for the Federal Interdepartmental Working Group on FGM. Copies of this report are available from: Women's Health Bureau Health Canada [email protected] The Canadian Women's Health Network 203-419 Graham Avenue Winnipeg, Manitoba R3C 0M3 fax: (204)989-2355 The opinions expressed in this report are not necessarily those of the Government of Canada or any of the other organizations represented. Dedication This report is dedicated to all the women in the world who have undergone FGM and to all the people who are helping them live with and reverse this procedure. This report is part of the ongoing commitment of Canadians and the Government of Canada to stop this practice in Canada and to improve the health and well-being of affected women and their communities. Executive Summary Female genital mutilation (FGM), or the ritual excision of part or all of the external female genitalia, is an ancient cultural practice that occurs around the world today, especially in Africa. With recent immigration to Canada of peoples from Somalia, Ethiopia and Eritrea, Sudan and Nigeria, women who have undergone this practice are now increasingly living in Canada. It is firmly believed by the people who practise it, that FGM improves feminine hygiene, that it will help eliminate disease and it is thought to be the only way to preserve family honour, a girl's virginity and her marriageability. FGM has a number of important adverse health effects including risks of infection and excessive bleeding (often performed when a girl is pre-pubertal). -

Agreement Between Endocervical Brush and Endocervical Curettage in Patients Undergoing Repeat Endocervical Sampling
Agreement Between Endocervical Brush and Endocervical Curettage in Patients Undergoing Repeat Endocervical Sampling Meredith J. Alston MD David W. Doo MD Sara E. Mazzoni MD MPH Elaine H. Stickrath MD Denver Health Medical Center University of Colorado Department of Obstetrics and Gynecology Background • Women with abnormal Pap tests referred for colposcopy frequently require sampling of the endocervix • ASCCP deems both the endocervical brush (ECB) and the endocervical currette (ECC) appropriate means of collecting endocervical samples (1) • Both have advantages and disadvantages, and it is unclear if one modality is superior Background • ECB has good sensitivity for endocervical lesions, it has poor specificity, ranging from 26- 38%2,3 • ECC has an excellent negative predictive value of 99.4% in women who had a satisfactory colposcopy • ECB is better tolerated by the patient, but runs the risk of contamination from the ectocervix • ECC is less likely to obtain an adequate sample Background • The results from the endocervical sample may influence clinical management after colposcopy. • Certain treatment options for high grade squamous intraepithelial neoplasia are available only to women with negative endocervical sampling. ▫ Ablative techniques ▫ Expectant management Background • Over concerns related to potential complications of excisional treatments, as well as over- treatment, there has been a push to re-introduce ablative techniques and, in appropriate clinical circumstances, expectant management, into the routine treatment of patients with cervical cancer precursors (12). Background • At our institution, it is our concern that due to the possibility of contamination from the ectocervix, a positive ECB may not represent a true positive endocervical sample. • We do not use a sleeve • Positive ECBs return for ECC if otherwise a candidate for ablative therapy or expectant management Objective • To describe the agreement between these two modalities of endocervical sampling. -

Gender Reassignment Surgery Policy Number: PG0311 ADVANTAGE | ELITE | HMO Last Review: 07/01/2021
Gender Reassignment Surgery Policy Number: PG0311 ADVANTAGE | ELITE | HMO Last Review: 07/01/2021 INDIVIDUAL MARKETPLACE | PROMEDICA MEDICARE PLAN | PPO GUIDELINES This policy does not certify benefits or authorization of benefits, which is designated by each individual policyholder terms, conditions, exclusions and limitations contract. It does not constitute a contract or guarantee regarding coverage or reimbursement/payment. Self-Insured group specific policy will supersede this general policy when group supplementary plan document or individual plan decision directs otherwise. Paramount applies coding edits to all medical claims through coding logic software to evaluate the accuracy and adherence to accepted national standards. This medical policy is solely for guiding medical necessity and explaining correct procedure reporting used to assist in making coverage decisions and administering benefits. SCOPE X Professional X Facility DESCRIPTION Transgender is a broad term that can be used to describe people whose gender identity is different from the gender they were thought to be when they were born. Gender dysphoria (GD) or gender identity disorder is defined as evidence of a strong and persistent cross-gender identification, which is the desire to be, or the insistence that one is of the other gender. Persons with this disorder experience a sense of discomfort and inappropriateness regarding their anatomic or genetic sexual characteristics. Individuals with GD have persistent feelings of gender discomfort and inappropriateness of their anatomical sex, strong and ongoing cross-gender identification, and a desire to live and be accepted as a member of the opposite sex. Gender Dysphoria (GD) is defined by the Diagnostic and Statistical Manual of Mental Disorders - Fifth Edition, DSM-5™ as a condition characterized by the "distress that may accompany the incongruence between one’s experienced or expressed gender and one’s assigned gender" also known as “natal gender”, which is the individual’s sex determined at birth. -

Colposcopy.Pdf
CCololppooscoscoppyy ► Chris DeSimone, M.D. ► Gynecologic Oncology ► Images from Colposcopy Cervical Pathology, 3rd Ed., 1998 HistoHistorryy ► ColColpposcopyoscopy wwasas ppiioneeredoneered inin GGeermrmaanyny bbyy DrDr.. HinselmannHinselmann dduriurinngg tthhee 19201920’s’s ► HeHe sousougghtht ttoo prprooveve ththaatt micmicrroscopicoscopic eexaminxaminaationtion ofof thethe cervixcervix wouwoulldd detectdetect cervicalcervical ccancanceerr eeararlliierer tthhaann 44 ccmm ► HisHis workwork identidentiifiefiedd severalseveral atatyypicalpical appeappeararanancceses whwhicichh araree stistillll usedused ttooddaay:y: . Luekoplakia . Punctation . Felderung (mosaicism) Colposcopy Cervical Pathology 3rd Ed. 1998 HistoHistorryy ► ThrThrooughugh thethe 3030’s’s aanndd 4040’s’s brbreaeaktkthrhrouougghshs wwereere mamaddee regregaarrddinging whwhicichh aapppepeararancanceess wweerere moremore liklikelelyy toto prprogogressress toto invinvaasivesive ccaarcinomrcinomaa;; HHOOWEWEVVERER,, ► TheThessee ffiinndingsdings wweerere didifffficiculultt toto inteinterrpretpret sincesince theythey werweree notnot corcorrrelatedelated wwithith histologhistologyy ► OneOne resreseaearcrchherer wwouldould claclaiimm hhiiss ppatatientsients wwithith XX ffindindiingsngs nevernever hahadd ccaarcinomarcinoma whwhililee aannothotheerr emphemphaatiticcallyally belibelieevedved itit diddid ► WorldWorld wiwidede colposcopycolposcopy waswas uunnderderuutitillizizeedd asas aa diadiaggnosticnostic tooltool sseeconcondadaryry ttoo tthheseese discrepadiscrepannciescies HistoHistorryy -

2021 HEDIS Reference Guidefor Primary Care
2021 HEDIS Reference Guide for Primary Care Cervical Cancer Screening (CCS) Patient Profile MVP members 21–64 years of age who have been screened for cervical cancer. Appropriate screening is defined by one of the following criteria: • Women 21–64 years of age who have a cervical cytology performed within the last three years (the measurement year and up to two years prior) • Women 30–64 years of age who have a cervical high-risk human papillomavirus (hrHPV) testing performed within the last five years (Note: Evidence of hrHPV testing within the last 5 years also captures patients who had cotesting; therefore additional methods to identify cotesting are not necessary.) • Women 30-64 years of age who had cervical cytology cotesting within the last five years Those excluded are women who have had a hysterectomy with no residual cervix (complete, total, or radical abdominal or vaginal hysterectomy), cervical agenesis, or acquired absence of cervix any time during the member's history through December 31 of the measurement year. How to Implement Best Practices and Improve Performance • Documentation in the medical record must include the name of the cervical screening, date of the test, and the result. This may be documented in an office note or a lab report, and can be submitted. • Cervical biopsies are not valid for primary cervical cancer screening and cannot be submitted. • When documenting medical/surgical history, avoid the use of “hysterectomy” alone, as this is not sufficient evidence that the cervix was removed. Be specific: “TAH”, TVH”, etc. • Documentation of “hysterectomy” alone in combination with documentation that the “patient no longer needs cervical cancer screening,” does meet criteria. -
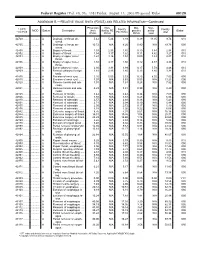
RELATIVE VALUE UNITS (RVUS) and RELATED INFORMATION—Continued
Federal Register / Vol. 68, No. 158 / Friday, August 15, 2003 / Proposed Rules 49129 ADDENDUM B.—RELATIVE VALUE UNITS (RVUS) AND RELATED INFORMATION—Continued Physician Non- Mal- Non- 1 CPT/ Facility Facility 2 MOD Status Description work facility PE practice acility Global HCPCS RVUs RVUs PE RVUs RVUs total total 42720 ....... ........... A Drainage of throat ab- 5.42 5.24 3.93 0.39 11.05 9.74 010 scess. 42725 ....... ........... A Drainage of throat ab- 10.72 N/A 8.26 0.80 N/A 19.78 090 scess. 42800 ....... ........... A Biopsy of throat ................ 1.39 2.35 1.45 0.10 3.84 2.94 010 42802 ....... ........... A Biopsy of throat ................ 1.54 3.17 1.62 0.11 4.82 3.27 010 42804 ....... ........... A Biopsy of upper nose/ 1.24 3.16 1.54 0.09 4.49 2.87 010 throat. 42806 ....... ........... A Biopsy of upper nose/ 1.58 3.17 1.66 0.12 4.87 3.36 010 throat. 42808 ....... ........... A Excise pharynx lesion ...... 2.30 3.31 1.99 0.17 5.78 4.46 010 42809 ....... ........... A Remove pharynx foreign 1.81 2.46 1.40 0.13 4.40 3.34 010 body. 42810 ....... ........... A Excision of neck cyst ........ 3.25 5.05 3.53 0.25 8.55 7.03 090 42815 ....... ........... A Excision of neck cyst ........ 7.07 N/A 5.63 0.53 N/A 13.23 090 42820 ....... ........... A Remove tonsils and ade- 3.91 N/A 3.63 0.28 N/A 7.82 090 noids.