COVID Vaccine Provider Bulletin Week of May 31, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dose Timing Calendar.Xlsx
APRIL 2021 COVID-19 Vaccine Dose Calendar Located the date of your first dose and it will show you the earliest date you can receive the boost dose for each brand. This calendar also provides the end of the reccomended window for boost doses which is 42 days after the first dose. If you are past the 42 day window you can still receive a boost dose. Please contact Lane County Public Health for more information 541-682-1380 Sunday Monday Tuesday Wednesday Thursday Friday Saturday Mar 28 Mar 29 Mar 30 Mar 31 Apr 1 Apr 2 Apr 3 Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Pfizer Apr 18 May 9 Pfizer Apr 19 May 10 Pfizer Apr 20 May 11 Pfizer Apr 21 May 12 Pfizer Apr 22 May 13 Pfizer Apr 23 May 14 Pfizer Apr 24 May 15 Moderna Apr 25 May 9 Moderna Apr 26 May 10 Moderna Apr 27 May 11 Moderna Apr 28 May 12 Moderna Apr 29 May 13 Moderna Apr 30 May 14 Moderna May 1 May 15 4 5 6 7 8 9 10 Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Pfizer Apr 25 May 16 Pfizer Apr 26 May 17 Pfizer Apr 27 May 18 Pfizer Apr 28 May 19 Pfizer Apr 29 May 20 Pfizer Apr 30 May 21 Pfizer May 1 May 22 Moderna May 2 May 16 Moderna May 3 May 17 Moderna May 4 May 18 Moderna May 5 May 19 Moderna May 6 May 20 Moderna May 7 May 21 Moderna May 8 May 22 11 12 13 14 15 16 17 Earliest Boost 42 days Earliest Boost 42 days Earliest Boost 42 days Earliest Boost -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2020-2021 Academic Year Grid ALL 11X17
Fall 2020 Spring 2021 Summer 2021* EVENTS / DEADLINES Session 1 Session 1 Session 2 Session 3 Session 4 Session 5 Session 6 Winter Mini Session 2 Session 3 Session 4 Session 5 Session 6 Summer Mini Session 1 Session 2 Session 3 Session 4 Regular Regular First Day of Classes September 28, October 19, November 2, December 21, February 22, *Please also see notes below August 24, 2020 August 24, 2020 August 24, 2020 January 19, 2021 January 19, 2021 January 19, 2021 March 22, 2021 April 5, 2021 May 17, 2021 June 7, 2021 June 7, 2021 June 7, 2021 July 12, 2021 2020 2020 2020 2020 2021 regarding college-specific dates and Monday Monday Monday Tuesday Tuesday Tuesday Monday Monday Monday Monday Monday Monday Monday Monday Monday Monday Monday Monday summer sessions meeting days. Labor Day Holiday (Fall); September 7, 2020 January 18, 2021 May 31, 2021 Martin Luther King Holiday (Spring); Monday Monday Monday Memorial Day (Summer) **Extended** September 30, October 21, November 4, December 22, February 24, Last Day to Add a Class September 1, August 26, 2020 August 26, 2020 January 26, 2021 January 21, 2021 January 21, 2021 March 24, 2021 April 7, 2021 May 18, 2021 June 8, 2021 June 8, 2021 June 8, 2021 July 13, 2021 2020 2020 2020 2020 2021 or be enrolled from the Wait List 2020 Wednesday Wednesday Tuesday Thursday Thursday Wednesday Wednesday Tuesday Tuesday Tuesday Tuesday Tuesday Wednesday Wednesday Wednesday Tuesday Wednesday Tuesday ORD - Official Reporting Day Last day to drop a course or withdraw without receiving a grade Last day to -

Federal Register/Vol. 86, No. 98/Monday, May 24, 2021/Notices
27904 Federal Register / Vol. 86, No. 98 / Monday, May 24, 2021 / Notices Dated: May 19, 2021. There are no meetings scheduled for POSTAL REGULATORY COMMISSION Crystal Robinson, the week of June 14, 2021. [Docket No. MC2021–91; Order No. 5894] Committee Management Officer. Week of June 21, 2021—Tentative [FR Doc. 2021–10903 Filed 5–21–21; 8:45 am] Mail Classification Schedule BILLING CODE 7555–01–P Tuesday, June 22, 2021 AGENCY: Postal Regulatory Commission. 9:00 a.m. Briefing on Transformation ACTION: Notice. at the NRC—Midyear Review NUCLEAR REGULATORY SUMMARY: The Commission is COMMISSION (Public Meeting). (Contact: Maria Arribas-Colon: 301–415–6026) acknowledging a recent Postal Service [NRC–2021–0001] filing concerning classification changes Additional Information: Due to to the Mail Classification Schedule Sunshine Act Meetings COVID–19, there will be no physical related to International Mail. This public attendance. The public is invited document informs the public of the TIME AND DATE: Weeks of May 24, 31, to attend the Commission’s meeting live filing, invites public comment, and June 7, 14, 21, 28, 2021. by webcast at the Web address—https:// takes other administrative steps. PLACE: Commissioners’ Conference video.nrc.gov/. DATES: Comments are due: May 25, Room, 11555 Rockville Pike, Rockville, Week of June 28, 2021—Tentative 2021. Maryland. ADDRESSES: Submit comments STATUS: Public. There are no meetings scheduled for electronically via the Commission’s MATTERS TO BE CONSIDERED: the week of June 28, 2021. Filing Online system at http:// Week of May 24, 2021 CONTACT PERSON FOR MORE INFORMATION: www.prc.gov. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

2020–2021District Calendar
2020–2021 DISTRICT CALENDAR August 10-14 ...........................New Leader Institute November 11 .................... Veterans Day: No school Major Religious & Cultural Holidays August 17-20 ........ BPS Learns Summer Conference November 25 ..... Early release for students and staff 2020 2021 (TSI, ALI, English Learner Symposium, November 26-27 ....Thanksgiving Recess: No school July 31 ..........Eid al-Adha Jan. 1 ........New Year’s Day Early Childhood/UPK Conference, December 24- January 1 ..Winter Recess: No school Sep. 19-20 ..Rosh Hashanah Jan. 6 ......Three Kings Day New Teacher Induction) January 4.................... All teachers and paras report . Sep. 28 .........Yom Kippur Feb. 12 .... Lunar New Year September 1 ......REMOTE: UP Academies: Boston, January 5...................... Students return from recess Nov. 14 ...... Diwali begins Feb. 17 ... Ash Wednesday Dorchester, and Holland, January 18..................M.L. King Jr. Day: No school all grades − first day of school Nov. 26 .......Thanksgiving Mar. 27-Apr. 3 ..... Passover February 15 .................... Presidents Day: No school September 7 .......................... Labor Day: No school Dec. 10-18 .......Hanukkah Apr. 2 ............ Good Friday February 16-19.............February Recess: No school September 8 .............. All teachers and paras report Dec. 25 ............Christmas Apr. 4 ...................... Easter April 2 ...................................................... No school September 21 ..........REMOTE: ALL Students report Dec. -
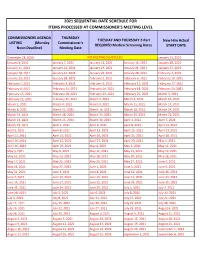
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

190 Public Law 89.437-May 31, 1966 [80 Stat
190 PUBLIC LAW 89.437-MAY 31, 1966 [80 STAT. Public Law 89-437 May 31, 1966 AN ACT [H. R. 12262] rj^^ continue until the close of June 30, 1969, the existing suspension of duty on certain copying shoe lathes. Be it enacted hy the Senate and Houae of Representatives of the Duty iulpln- United States of America m Congress assembled, That (a) item 911.70 sion. of the Tariff Schedules of the United States (19 LT.S.C, sec. 1202, item 7 8^sfaf 231''^'*' -^ll-^O) is amended by striking out "On or before 6/30/66" and insert ing in lieu thereof "On or before 6/80/69". (b) The amendment made by subsection (a) shall apply with respect to articles entered, or withdrawn from warehouse, for con sumption, after June 30,1966. Approved May 31, 1966. Public Law 89-438 May 31, 1966 AN ACT [H. R. 103 66] rp^y establish the Mount Rogers National Recreation Area in the Jefferson National Forest in Virginia, and for other purposes. Be it enacted by the Senate and House of Representatives of the Mount Rogers United States of America in Congress assembled. That, in order to tion Area, Va. provide for the public outdoor recreation use and enjoyment of the area in the vicinity of Mount Rogers, the highest mountain in the State of Virginia, and to the extent feasible the conservation of scenic, scien tific, historic, and other values of the area, the Secretary of Agriculture shall establish the Mount Rogers National Recreation Area in the Jefferson National Forest in the State of Virginia. -

Chapter 7:Financial Statements for a Proprietorship: Chapter Overview Chapter Overview
Chapter 7:Financial Statements for a Proprietorship: Chapter Overview Chapter Overview Financial Statements for a Proprietorship: Chapter Objectives Financial Statements for a Proprietorship: Accounting in the Real World Financial Statements for a Proprietorship: Key Terms Financial Statements for a Proprietorship: Chapter Objectives Learning Objectives After studying Chapter 7, in addition to defining key terms, you will be able to: LO1 Prepare an income statement for a service business. LO2 Calculate and analyze financial ratios using income statement amounts. LO3 Prepare a balance sheet for a service business organized as a proprietorship. Financial Statements for a Proprietorship: Accounting in the Real World American Eagle Outfitters If you have visited a shopping mall, you have probably seen at least one of the stores belonging to the American Eagle Outfitters (AEO) family. AEO markets its merchandise through three stores: American Eagle Outfitters, Aerie, and 77Kids. AEO “offers high-quality, on-trend clothing, accessories and personal care products at affordable prices.” AEO brand targets 15- to 25-year-olds and has over 900 stores in the United States and Canada. The AEO website states, “Since our first store opened in 1977, AEO has focused on innovation.” One way AEO is trying to maintain its focus on innovation is by hiring young, creative new employees. The company is taking its motto (Live Your Life) one step further with a motto of “Live Your Life, Love Your Job” for employees and potential employees. In a campaign to attract new employees, AEO uses three methods: internships, full-time training programs, and campus visits. College juniors can complete a paid summer internship at AEO's corporate office. -

114-Day Swine Gestation Table
114-DAY SWINE GESTATION TABLE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE Jan 1 Apr 25 Mar 1 Jun 23 May 1 Aug 23 Jul 1 Oct 23 Sep 1 Dec 24 Nov 1 Feb 23 Jan 2 Apr 26 Mar 2 Jun 24 May 2 Aug 24 Jul 2 Oct 24 Sep 2 Dec 25 Nov 2 Feb 24 Jan 3 Apr 27 Mar 3 Jun 25 May 3 Aug 25 Jul 3 Oct 25 Sep 3 Dec 26 Nov 3 Feb 25 Jan 4 Apr 28 Mar 4 Jun 26 May 4 Aug 26 Jul 4 Oct 26 Sep 4 Dec 27 Nov 4 Feb 26 Jan 5 Apr 29 Mar 5 Jun 27 May 5 Aug 27 Jul 5 Oct 27 Sep 5 Dec 28 Nov 5 Feb 27 Jan 6 Apr 30 Mar 6 Jun 28 May 6 Aug 28 Jul 6 Oct 28 Sep 6 Dec 29 Nov 6 Feb 28 Jan 7 May 1 Mar 7 Jun 29 May 7 Aug 29 Jul 7 Oct 29 Sep 7 Dec 30 Nov 7 Mar 1 Jan 8 May 2 Mar 8 Jun 30 May 8 Aug 30 Jul 8 Oct 30 Sep 8 Dec 31 Nov 8 Mar 2 Jan 9 May 3 Mar 9 Jul 1 May 9 Aug 31 Jul 9 Oct 31 Sep 9 Jan 1 Nov 9 Mar 3 Jan 10 May 4 Mar 10 Jul 2 May 10 Sep 1 Jul 10 Nov 1 Sep 10 Jan 2 Nov 10 Mar 4 Jan 11 May 5 Mar 11 Jul 3 May 11 Sep 2 Jul 11 Nov 2 Sep 11 Jan 3 Nov 11 Mar 5 Jan 12 May 6 Mar 12 Jul 4 May 12 Sep 3 Jul 12 Nov 3 Sep 12 Jan 4 Nov 12 Mar 6 Jan 13 May 7 Mar 13 Jul 5 May 13 Sep 4 Jul 13 Nov 4 Sep 13 Jan 5 Nov 13 Mar 7 Jan 14 May 8 Mar 14 Jul 6 May 14 Sep 5 Jul 14 Nov 5 Sep 14 Jan 6 Nov 14 Mar 8 Jan 15 May 9 Mar 15 Jul 7 May 15 Sep 6 Jul 15 Nov 6 Sep 15 Jan 7 Nov 15 Mar 9 Jan 16 May 10 Mar 16 Jul 8 May 16 Sep 7 Jul 16 Nov 7 Sep 16 Jan 8 Nov 16 Mar 10 Jan 17 May 11 Mar 17 Jul 9 May 17 Sep 8 Jul 17 Nov 8 Sep 17 Jan 9 Nov 17 Mar 11 Jan 18 May 12 Mar 18 Jul 10 May 18 Sep 9 Jul 18 Nov 9 Sep 18 Jan 10 Nov -

Sweeping Schedule FY21.Xlsx
City of Bowie Street Sweeping Schedule March - October 2021 Streets will be swept approximately every 8 weeks. (Please remove cars from the street when sweeping is scheduled in your neighborhood) Sweeping done the week of: Adnell Woods (City Portion) May 17, July 19, September 13 Allen Pond April 26, June 21, August 23, October 18 Amber Meadows April 19, June 14, August 16, October 11 Ashleigh April 19, June 14, August 16, October 11 Ashleigh Station April 19, June 14, August 16, October 11 Belair Greens May 3, June 28, August 30, October 25 Belair Town 1 May 3, June 28, August 30, October 25 Belair Town II May 24, July 26, September 20 Buckingham May 3, June 28, August 30, October 25 Chapel Forge May 24, July 26, September 20 Collington Manor April 26, June 21, August 23, October 18 Collington Station May 17, July 19, September 13 Covington Knolls April 26, June 21, August 23, October 18 Covington Manor April 26, June 21, August 23, October 18 Derbyshire May 3, June 28, August 30, October 25 Devonshire May 17, July 19, September 13 Dixons Crossing May 17, July 19, September 13 Enfield April 26, June 21, August 23, October 18 Enfield Chase Townhomes** April 26, June 21, August 23, October 18 Ensleigh April 5, May 31, August 2, September 27 Essington April 5, May 31, August 2, September 27 Fairview May 17, July 19, September 13 Foxhill April 5, May 31, August 2, September 27 Gateway/ Renaissance April 5, May 31, August 2, September 27 Glen Allen April 26, June 21, August 23, October 18 Gradys Walk May 17, July 19, September 13 Greystone** -

Resort-Calendar.Pdf
Week 2020 2021 2022 2023 2024 no 1 10-Jan - 17-Jan 8-Jan - 15-Jan 7-Jan - 14-Jan 6-Jan - 13-Jan 5-Jan - 12-Jan 2 17-Jan - 24-Jan 15-Jan - 22-Jan 14-Jan - 21-Jan 13-Jan - 20-Jan 12-Jan - 19-Jan 3 24-Jan - 31-Jan 22-Jan - 29-Jan 21-Jan - 28-Jan 20-Jan - 27-Jan 19-Jan - 26-Jan 4 31-Jan - 7-Feb 29-Jan - 5-Feb 28-Jan - 4-Feb 27-Jan - 3-Feb 26-Jan - 2-Feb 5 7-Feb - 14-Feb 5-Feb - 12-Feb 4-Feb - 11-Feb 3-Feb - 10-Feb 2-Feb - 9-Feb 6 14-Feb - 21-Feb 12-Feb - 19-Feb 11-Feb - 18-Feb 10-Feb - 17-Feb 9-Feb - 16-Feb 7 21-Feb - 28-Feb 19-Feb - 26-Feb 18-Feb - 25-Feb 17-Feb - 24-Feb 16-Feb - 23-Feb 8 28-Feb - 6-Mar 26-Feb - 5-Mar 25-Feb - 4-Mar 24-Feb - 3-Mar 23-Feb - 1-Mar 9 6-Mar - 13-Mar 5-Mar - 12-Mar 4-Mar - 11-Mar 3-Mar - 10-Mar 1-Mar - 8-Mar 10 13-Mar - 20-Mar 12-Mar - 19-Mar 11-Mar - 18-Mar 10-Mar - 17-Mar 8-Mar - 15-Mar 11 20-Mar - 27-Mar 19-Mar - 26-Mar 18-Mar - 25-Mar 17-Mar - 24-Mar 15-Mar - 22-Mar 12 27-Mar - 3-Apr 26-Mar - 2-Apr 25-Mar - 1-Apr 24-Mar - 31-Mar 22-Mar - 29-Mar 13 3-Apr - 10-Apr 2-Apr - 9-Apr 1-Apr - 8-Apr 31-Mar - 7-Apr 29-Mar - 5-Apr 14 10-Apr - 17-Apr 9-Apr - 16-Apr 8-Apr - 15-Apr 7-Apr - 14-Apr 5-Apr - 12-Apr 15 17-Apr - 24-Apr 16-Apr - 23-Apr 15-Apr - 22-Apr 14-Apr - 21-Apr 12-Apr - 19-Apr 16 24-Apr - 1-May 23-Apr - 30-Apr 22-Apr - 29-Apr 21-Apr - 28-Apr 19-Apr - 26-Apr 17 1-May - 8-May 30-Apr - 7-May 29-Apr - 6-May 28-Apr - 5-May 26-Apr - 3-May 18 8-May - 15-May 7-May - 14-May 6-May - 13-May 5-May - 12-May 3-May - 10-May 19 15-May - 22-May 14-May - 21-May 13-May - 20-May 12-May - 19-May 10-May - 17-May 20 22-May - 29-May