Submitted to Icarus on August 4, 2021 1 Organic Carbon Cycle In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Radiation Forces on Small Particles in the Solar System T
1CARUS 40, 1-48 (1979) Radiation Forces on Small Particles in the Solar System t JOSEPH A. BURNS Cornell University, 2 Ithaca, New York 14853 and NASA-Ames Research Center PHILIPPE L. LAMY CNRS-LAS, Marseille, France 13012 AND STEVEN SOTER Cornell University, Ithaca, New York 14853 Received May 12, 1978; revised May 2, 1979 We present a new and more accurate expression for the radiation pressure and Poynting- Robertson drag forces; it is more complete than previous ones, which considered only perfectly absorbing particles or artificial scattering laws. Using a simple heuristic derivation, the equation of motion for a particle of mass m and geometrical cross section A, moving with velocity v through a radiation field of energy flux density S, is found to be (to terms of order v/c) mi, = (SA/c)Qpr[(1 - i'/c)S - v/c], where S is a unit vector in the direction of the incident radiation,/" is the particle's radial velocity, and c is the speed of light; the radiation pressure efficiency factor Qpr ~ Qabs + Q~a(l - (cos a)), where Qabs and Q~c~ are the efficiency factors for absorption and scattering, and (cos a) accounts for the asymmetry of the scattered radiation. This result is confirmed by a new formal derivation applying special relativistic transformations for the incoming and outgoing energy and momentum as seen in the particle and solar frames of reference. Qpr is evaluated from Mie theory for small spherical particles with measured optical properties, irradiated by the actual solar spectrum. Of the eight materials studied, only for iron, magnetite, and graphite grains does the radiation pressure force exceed gravity and then just for sizes around 0. -

Guest Editorial: My Formative Years with the Rasc by Sara Seager I First Learned About Astronomy As a Small Child
INTRODUCTION 9 GUEST EDITORIAL: MY FORMATIVE YEARS WITH THE RASC BY SARA SEAGER I first learned about astronomy as a small child. One of my first memories is looking through a telescope at the Moon. I was completely stunned by what I saw. The Moon— huge and filled with craters—was a world in and of itself. I was with my father, at a star party hosted by the RASC. Later, when I was 10 years old and on my first camping trip, I remember awakening late one night, stepping outside the tent, and looking up. Stars—millions of them it seemed—filled the entire sky and took my breath away. As a teenager in the late 1980s, I joined the RASC Toronto Centre and deter- minedly attended the bimonthly Friday night meetings held in what was then the Planetarium building. Much of the information went over my head, but I can still clearly remember the excitement of the group whenever a visiting professor presented on a novel topic. An RASC member offered a one-semester evening class on astron- omy, and we were able to use the planetarium to learn about the night sky. The RASC events were a highlight of my high school and undergraduate years. While majoring in math and physics at the University of Toronto, I was thrilled to intern for two summers at the David Dunlap Observatory (DDO). I carried out an observing program of variable stars (including Polaris, the North Star), using the 61-cm (24″) and 48-cm (19″) telescopes atop the DDO’s administration building for simultaneous photometry of the target and comparison stars. -

Planetary Defence Activities Beyond NASA and ESA
Planetary Defence Activities Beyond NASA and ESA Brent W. Barbee 1. Introduction The collision of a significant asteroid or comet with Earth represents a singular natural disaster for a myriad of reasons, including: its extraterrestrial origin; the fact that it is perhaps the only natural disaster that is preventable in many cases, given sufficient preparation and warning; its scope, which ranges from damaging a city to an extinction-level event; and the duality of asteroids and comets themselves---they are grave potential threats, but are also tantalising scientific clues to our ancient past and resources with which we may one day build a prosperous spacefaring future. Accordingly, the problems of developing the means to interact with asteroids and comets for purposes of defence, scientific study, exploration, and resource utilisation have grown in importance over the past several decades. Since the 1980s, more and more asteroids and comets (especially the former) have been discovered, radically changing our picture of the solar system. At the beginning of the year 1980, approximately 9,000 asteroids were known to exist. By the beginning of 2001, that number had risen to approximately 125,000 thanks to the Earth-based telescopic survey efforts of the era, particularly the emergence of modern automated telescopic search systems, pioneered by the Massachusetts Institute of Technology’s (MIT’s) LINEAR system in the mid-to-late 1990s.1 Today, in late 2019, about 840,000 asteroids have been discovered,2 with more and more being found every week, month, and year. Of those, approximately 21,400 are categorised as near-Earth asteroids (NEAs), 2,000 of which are categorised as Potentially Hazardous Asteroids (PHAs)3 and 2,749 of which are categorised as potentially accessible.4 The hazards posed to us by asteroids affect people everywhere around the world. -

Me Gustaría Dirigir Una Misión 'Starshade' Para Encontrar Un Gemelo De La Tierra
CIENCIAS SARA SEAGER, PROFESORA DE FÍSICA Y CIENCIA PLANETARIA EN EL MIT “Me gustaría dirigir una misión ‘starshade’ para encontrar un gemelo de la Tierra” Esta astrofísica del Instituto Tecnológico de Massachusetts (EE UU) está empeñada en observar un planeta extrasolar parecido al nuestro y, para poderlo ver, su sueño es desplegar un enorme ‘girasol’ en el espacio que oculte la cegadora luz de su estrella anfitriona. Es una de las aventuras en las que está embarcada y que cuenta en su último libro, Las luces más diminutas del universo, una historia de amor, dolor y exoplanetas. Enrique Sacristán 30/7/2021 08:30 CEST Sara Seager y uno de los pósteres de exoplanetas (facilitados por el Jet Propulsion Laboratory) que adornan el pasillo de su despacho en el Instituto Tecnológico de Massachusetts. / MIT/JPL- NASA Con tan solo diez años quedó deslumbrada por el cielo estrellado durante una acampada, una experiencia que recomienda a todo el mundo: “Este año la lluvia de meteoros de las perseidas, alrededor del 12 de agosto, cae cerca de una luna nueva, por lo que el cielo estará especialmente oscuro y habrá buenas condiciones para observar las estrellas fugaces”, nos adelanta la astrofísica Sara Seager. Nació en julio de 1971 en Toronto (Canadá) y hoy es una experta mundial en la búsqueda de exoplanetas, en especial los similares a la Tierra y con CIENCIAS indicios de vida. Se graduó en su ciudad natal y se doctoró en Harvard; luego pasó por el Instituto de Estudios Avanzados en Princeton y el Instituto Carnegie de Washington; hasta que en 2007 se asentó como profesora en el Instituto Tecnológico de Massachusetts (MIT). -

Asteroid Retrieval Feasibility Study
Asteroid Retrieval Feasibility Study 2 April 2012 Prepared for the: Keck Institute for Space Studies California Institute of Technology Jet Propulsion Laboratory Pasadena, California 1 2 Authors and Study Participants NAME Organization E-Mail Signature John Brophy Co-Leader / NASA JPL / Caltech [email protected] Fred Culick Co-Leader / Caltech [email protected] Co -Leader / The Planetary Louis Friedman [email protected] Society Carlton Allen NASA JSC [email protected] David Baughman Naval Postgraduate School [email protected] NASA ARC/Carnegie Mellon Julie Bellerose [email protected] University Bruce Betts The Planetary Society [email protected] Mike Brown Caltech [email protected] Michael Busch UCLA [email protected] John Casani NASA JPL [email protected] Marcello Coradini ESA [email protected] John Dankanich NASA GRC [email protected] Paul Dimotakis Caltech [email protected] Harvard -Smithsonian Center for Martin Elvis [email protected] Astrophysics Ian Garrick-Bethel UCSC [email protected] Bob Gershman NASA JPL [email protected] Florida Institute for Human and Tom Jones [email protected] Machine Cognition Damon Landau NASA JPL [email protected] Chris Lewicki Arkyd Astronautics [email protected] John Lewis University of Arizona [email protected] Pedro Llanos USC [email protected] Mark Lupisella NASA GSFC [email protected] Dan Mazanek NASA LaRC [email protected] Prakhar Mehrotra Caltech [email protected] -

Constraints on a Potential Aerial Biosphere on Venus: I. Cosmic Rays ⇑ Lewis R
Icarus 257 (2015) 396–405 Contents lists available at ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus Constraints on a potential aerial biosphere on Venus: I. Cosmic rays ⇑ Lewis R. Dartnell a, , Tom Andre Nordheim b,c, Manish R. Patel d,e, Jonathon P. Mason d, Andrew J. Coates b,c, Geraint H. Jones b,c a Space Research Centre, Department of Physics and Astronomy, University of Leicester, Leicester LE1 7RH, UK b Mullard Space Science Laboratory, University College London, Dorking, Surrey RH5 6NT, UK c Centre for Planetary Sciences at UCL/Birkbeck, University College London, Gower Street, London WC1E 6BT, UK d Department of Physical Sciences, The Open University, Milton Keynes, UK e Space Science and Technology Department, STFC Rutherford Appleton Laboratory, Chilton, Oxfordshire OX11 0QX, UK article info abstract Article history: While the present-day surface of Venus is certainly incompatible with terrestrial biology, the planet may Received 31 October 2014 have possessed oceans in the past and provided conditions suitable for the origin of life. Venusian life Revised 10 April 2015 may persist today high in the atmosphere where the temperature and pH regime is tolerable to terrestrial Accepted 7 May 2015 extremophile microbes: an aerial habitable zone. Here we argue that on the basis of the combined Available online 15 May 2015 biological hazard of high temperature and high acidity this habitable zone lies between 51 km (65 °C) and 62 km (À20 °C) altitude. Compared to Earth, this potential venusian biosphere may be exposed to Keywords: substantially more comic ionising radiation: Venus has no protective magnetic field, orbits closer to Astrobiology the Sun, and the entire habitable region lies high in the atmosphere – if this narrow band is sterilised Cosmic rays Exobiology there is no reservoir of deeper life that can recolonise afterwards. -

Risk Evaluation for 1-Bromopropane (N-Propyl Bromide) CASRN: 106-94-5
United States EPA Document #740-R1-8013 Environmental Protection Agency August 2020 Office of Chemical Safety and Pollution Prevention Risk Evaluation for 1-Bromopropane (n-Propyl Bromide) CASRN: 106-94-5 August 2020 TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................. 2 LIST OF TABLES ...................................................................................................................... 10 LIST OF APPENDIX TABLES ................................................................................................ 17 LIST OF FIGURES .................................................................................................................... 17 LIST OF APPENDIX FIGURES .............................................................................................. 19 LIST OF EQUATIONS .............................................................................................................. 19 LIST OF APPENDIX EQUATIONS ........................................................................................ 20 ACKNOWLEDGEMENTS ....................................................................................................... 21 ABBREVIATIONS ..................................................................................................................... 22 EXECUTIVE SUMMARY ........................................................................................................ 30 1 INTRODUCTION................................................................................................................... -

Exoplanet Exploration Collaboration Initiative TP Exoplanets Final Report
EXO Exoplanet Exploration Collaboration Initiative TP Exoplanets Final Report Ca Ca Ca H Ca Fe Fe Fe H Fe Mg Fe Na O2 H O2 The cover shows the transit of an Earth like planet passing in front of a Sun like star. When a planet transits its star in this way, it is possible to see through its thin layer of atmosphere and measure its spectrum. The lines at the bottom of the page show the absorption spectrum of the Earth in front of the Sun, the signature of life as we know it. Seeing our Earth as just one possibly habitable planet among many billions fundamentally changes the perception of our place among the stars. "The 2014 Space Studies Program of the International Space University was hosted by the École de technologie supérieure (ÉTS) and the École des Hautes études commerciales (HEC), Montréal, Québec, Canada." While all care has been taken in the preparation of this report, ISU does not take any responsibility for the accuracy of its content. Electronic copies of the Final Report and the Executive Summary can be downloaded from the ISU Library website at http://isulibrary.isunet.edu/ International Space University Strasbourg Central Campus Parc d’Innovation 1 rue Jean-Dominique Cassini 67400 Illkirch-Graffenstaden Tel +33 (0)3 88 65 54 30 Fax +33 (0)3 88 65 54 47 e-mail: [email protected] website: www.isunet.edu France Unless otherwise credited, figures and images were created by TP Exoplanets. Exoplanets Final Report Page i ACKNOWLEDGEMENTS The International Space University Summer Session Program 2014 and the work on the -

Problems with Panspermia Or Extraterrestrial Origin of Life Scenarios As Found on the IDEA Center Website At
Problems with Panspermia or Extraterrestrial Origin of Life Scenarios As found on the IDEA Center website at http://www.ideacenter.org Panspermia is the theory that microorganisms or biochemical compounds from outer space are responsible for originating life on Earth and possibly in other parts of the universe where suitable atmospheric conditions exist. Essentially, it is a hypothesis which states that life on earth came from outer space. It has been popularized in countless science fiction works, however some scientists, including the discoverer of the structure of DNA, Francis Crick, have advocated panspermia. There are generally about 3 different "flavors" of panspermia: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. 2. Directed Panspermia where intelligent life on other planets intentionally seeded other planets with their own form of life. 3. Intelligent Design Panspermia, where intelligent beings from another planet came to earth and designed life here. Each type of panspermia will be briefly discussed below: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. Naturalistic panspermia has gained popularity because some have recognized that life on earth appears very soon after the origin of conditions which would allow it to exist. Many scientists believed that if life had originated on earth it would have taken many hundreds of millions, or even billions of years to do so. Life appears perhaps less than 200 million after the origin of conditions at which life could exist. -
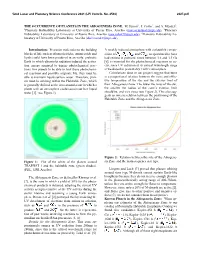
The Occurrence of Planets in the Abiogenesis Zone
52nd Lunar and Planetary Science Conference 2021 (LPI Contrib. No. 2548) 2327.pdf THE OCCURRENCE OF PLANETS IN THE ABIOGENESIS ZONE. M. Jusino1 , J. Colón2 , and A. Méndez3, 1Planetary Habitability Laboratory at University of Puerto Rico, Arecibo ([email protected]), 2Planetary Habitability Laboratory at University of Puerto Rico, Arecibo ([email protected]), 3Planetary Habitability La- boratory at University of Puerto Rico, Arecibo ([email protected]) Introduction: Precursor molecules to the building A weakly reduced atmosphere with a plausible compo- blocks of life such as ribonucleotides, amino acids and sition of , , , and , as speculated to have lipids could have been produced in an early, prebiotic had existed in prebiotic times between 3.8 and 3.5 Ga Earth in which ultraviolet radiation induced the activa- [6], is essential for the photochemical reactions to oc- tion energy required to trigger photochemical reac- cur, since UV radiation at its critical wavelength range tions. For planets to be able to host these photochemi- is weakened in present-day Earth’s atmosphere. cal reactions and possibly originate life, they must be Calculations done in our project suggest that there able to maintain liquid surface water. Therefore, plan- is a proportional relation between the mass and effec- ets must be orbiting within the Habitable Zone, which tive temperature of the star and the exterior limit of is generally defined as the area around a star in which a their Abiogenesis Zone. The lower the mass of the star, planet with an atmosphere could sustain surface liquid the smaller the radius of the zone’s exterior limit water [1]. -

Carl Sagan: the People’S Astronomer by David Morrison, NASA, Ames Research Center
Carl Sagan: The People’s Astronomer by David Morrison, NASA, Ames Research Center Introduction Carl Sagan was the world’s best known scientist in the late 20th century, serving as our guide to the planets during the golden age of solar system exploration. He was both a visionary and a committed defender of rational scientific thinking. For a time, he transcended the usual categories of academics to become a true celebrity. His life illustrates both the advantages (wealth, fame, access to the seats of power) and burdens (loss of privacy, stress, criticism from academic colleagues) this status implies. Sagan was propelled on his academic and public careers by a wealth of talent, a large share of good luck, and an intensely focused drive to succeed. His lifelong quest was to understand the universe, especially our planetary system, and to communicate the thrill of scientific discovery to others. A natural teacher, he loved to explain things and never made a questioner feel stupid for asking. Although Sagan had broad intellectual interests, his pursuit of his career left little time for other activities: he did not play golf or follow sports, take up painting or cooking or photography, sing or play a musical instrument, join a church or synagogue, or watch much television or movies. His first two wives complained that he devoted insufficient time to his marriage or his children (1). It is perhaps a matter of personal taste whether we attribute this drive to personal ego or a genuine commitment to educate and inspire people about science. Undoubtedly there were elements of both motivations present. -

Francis Crick, Cross-Worlds Influencer: a Narrative Model to Historicize Big Bioscience
King’s Research Portal DOI: 10.1016/j.shpsc.2015.08.003 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Aicardi, C. (2016). Francis Crick, cross-worlds influencer: A narrative model to historicize big bioscience. Studies in History and Philosophy of Science Part C: Studies in History and Philosophy of Biological and Biomedical Sciences, 55, 83-95. https://doi.org/10.1016/j.shpsc.2015.08.003 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim.