Tympanocryptis Pinguicolla) Following Its Rediscovery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Relationships and Subgeneric Taxonomy of Toad�Headed Agamas Phrynocephalus (Reptilia, Squamata, Agamidae) As Determined by Mitochondrial DNA Sequencing E
ISSN 00124966, Doklady Biological Sciences, 2014, Vol. 455, pp. 119–124. © Pleiades Publishing, Ltd., 2014. Original Russian Text © E.N. Solovyeva, N.A. Poyarkov, E.A. Dunayev, R.A. Nazarov, V.S. Lebedev, A.A. Bannikova, 2014, published in Doklady Akademii Nauk, 2014, Vol. 455, No. 4, pp. 484–489. GENERAL BIOLOGY Phylogenetic Relationships and Subgeneric Taxonomy of ToadHeaded Agamas Phrynocephalus (Reptilia, Squamata, Agamidae) as Determined by Mitochondrial DNA Sequencing E. N. Solovyeva, N. A. Poyarkov, E. A. Dunayev, R. A. Nazarov, V. S. Lebedev, and A. A. Bannikova Presented by Academician Yu.Yu. Dgebuadze October 25, 2013 Received October 30, 2013 DOI: 10.1134/S0012496614020148 Toadheaded agamas (Phrynocephalus) is an essen Trapelus, and Stellagama) were used in molecular tial element of arid biotopes throughout the vast area genetic analysis. In total, 69 sequences from the Gen spanning the countries of Middle East and Central Bank were studied, 28 of which served as outgroups (the Asia. They constitute one of the most diverse genera of members of Agamidae, Chamaeleonidae, Iguanidae, the agama family (Agamidae), variously estimated to and Lacertidae). comprise 26 to 40 species [1]. The subgeneric Phryno The fragment sequences of the following four cephalus taxonomy is poorly studied: recent taxo mitochondrial DNA genes were used in phylogenetic nomic revision have been conducted without analysis analysis: the genes of subunit I of cytochrome c oxi of the entire genus diversity [1]; therefore, its phyloge dase (COI), of subunits II and IV of NADHdehydro netic position within Agamidae family remains genase (ND2 and ND4), and of cytochrome b (cyt b). -

Nomenclature of Vertebrate Fauna
Nomenclature of Vertebrate Fauna Rebecca D. Bray Department of Terrestrial Zoology, WA Museum What is the WA Museum? How to keep updated on species names? Good Identification Collaboration between Industry and the WAM Public enquiries WAM field trips Industry & Government Nomenclature WAM Collections WAM databases Outreach Research Displays Surveys Talks/lectures Media interviews Atlas of Living Australia Taxonomy Training GBIF NatureMap Species discovery Publications Advice to government & industry Public enquiries WAM field trips Industry & Government Nomenclature WAM Collections WAM databases Outreach Research Displays Surveys Talks/lectures Media interviews Atlas of Living Australia Taxonomy Training GBIF NatureMap Species discovery Publications Advice to government & industry WA Museum Collections • Entire WAM collections: 4.5 million objects • AZ + TZ = $91 million • AZ + TZ holotypes = $11 million • Continual improvement and enhancement • Field trips, public submissions, industry • Many databased, but many are not…. – Location, coordinates – Date – Collectors name – Habitat – Identification Systematics The branch of biology which deals with classification and nomenclature. “WHAT IS IT”? Systematics “The classification of living organisms into hierarchical series of groups emphasizing their phylogenetic relationships” Lincoln, R.J., Boxshall, G.A. and Clark, P. F. 1982. A dictionary of ecology, evolution and systematics. Cambridge University Press Taxonomy “The theory and practice of describing, naming and classifying organisms” -
How Can I Find a JP
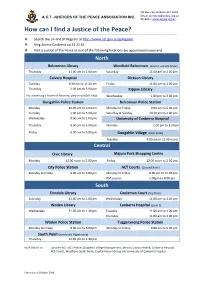
PO Box 766, Dickson ACT 2602 A.C.T. JUSTICES OF THE PEACE ASSOCIATION INC. Email: [email protected] Website: www.actjpa.org.au How can I find a Justice of the Peace? ❖ Search the on-line JP Register at http://www.act.gov.au/jpregister ❖ Ring Access Canberra on 13 22 81 ❖ Visit a Justice of the Peace at one of the following locations (no appointment necessary) North Belconnen Library Westfield Belconnen (level 3, outside Myer) Thursday 11.00 am to 1.00 pm Saturday 11.00 am to 1.00 pm Calvary Hospital Dickson Library Tuesday 9.30 am to 11.30 am Friday 11.00 am to 1.00 pm Thursday 1.00 pm to 3.00 pm Kippax Library For witnessing a Power of Attorney, please call 6201 6646. Wednesday 1.00 pm to 3.00 pm Gungahlin Police Station Belconnen Police Station Monday 10.00 am to 2.00 pm Monday to Friday 9.00 am to 5.00 pm Tuesday 1.00 pm to 5.00 pm Saturday & Sunday 10.30 am to 2.00 pm Wednesday 9.00 am to 1.00 pm University of Canberra Hospital Thursday 9.00 am to 5.00 pm Monday 1.00 pm to 3.00 pm Friday 9.00 am to 5.00 pm Gungahlin Village (near Coles) Tuesday 9.00 am to 12.00 noon Central Civic Library Majura Park Shopping Centre Monday 12.00 noon to 2.00 pm Friday 12.00 noon to 2.00 pm City Police Station ACT Courts (Ground floor) Monday to Friday 9.00 am to 5.00 pm Monday to Friday 9.30 am to 12.30 pm PM session 1.00pm to 4.00 pm . -

MAJURA PRIMARY SCHOOL Plan Your Trip with the TC Journey Planner! Visit Transport.Act.Gov.Au for Details Effective 20 July 2020
MAJURA PRIMARY SCHOOL Plan your trip with the TC Journey planner! Visit transport.act.gov.au for details Effective 20 July 2020 Local area map School start 9:00 am S im p s School finish 3:00 pm Bus Stop 3248 o n Route R9 to Belconnen S S t Knox St im p s o n S Bus stop location t 50 Regular route number Wade St Majura R9 RAPID route number A'Beckett St Primary School Walking route Bus Stop 3247 Irvine St Route R9 to Watson Burton St Knox St Watson Pl Williams St Bus Stop 3167 Bus Stop 3169 Routes R9 , 50 Bus Stop 3166 Route 50 to Watson to Watson Route 50 to City Windeyer StRoute R9 to Belconnen Local area map provides indicative Bus Stop 3168 Route 50 to City walking paths only. This map is designed to represent local public transport options in the area and is not to scale Summary of bus services Route AM PM Suburbs/Areas Serviced Other information R9 Belconnen Interchange, Bruce, Dickson Interchange, Watson 50 Watson, Downer, Dickson, Dickson Interchange, Lyneham, O'Connor, City Interchange transport.act.gov.au MAJURA PRIMARY SCHOOL Plan your trip with the TC Journey planner! Visit transport.act.gov.au for details Effective 20 July 2020 Morning regular services Other times available. Visit transport.act.gov.au 50 From City 50 From Watson City InterchangeMiller/ Lyneham Dickson Dickson ShopsWatson Watson Watson Platform 12 Hack Shops Interchange Plt(Cowper 1 St) Shops Terminus Shops 8:04 8:13 8:18 8:25 8:27 8:32 8:32 8:39 8:24 8:33 8:38 8:45 8:47 8:52 Morning RAPID services Other times available. -

An Annotated Type Catalogue of the Dragon Lizards (Reptilia: Squamata: Agamidae) in the Collection of the Western Australian Museum Ryan J
RECORDS OF THE WESTERN AUSTRALIAN MUSEUM 34 115–132 (2019) DOI: 10.18195/issn.0312-3162.34(2).2019.115-132 An annotated type catalogue of the dragon lizards (Reptilia: Squamata: Agamidae) in the collection of the Western Australian Museum Ryan J. Ellis Department of Terrestrial Zoology, Western Australian Museum, Locked Bag 49, Welshpool DC, Western Australia 6986, Australia. Biologic Environmental Survey, 24–26 Wickham St, East Perth, Western Australia 6004, Australia. Email: [email protected] ABSTRACT – The Western Australian Museum holds a vast collection of specimens representing a large portion of the 106 currently recognised taxa of dragon lizards (family Agamidae) known to occur across Australia. While the museum’s collection is dominated by Western Australian species, it also contains a selection of specimens from localities in other Australian states and a small selection from outside of Australia. Currently the museum’s collection contains 18,914 agamid specimens representing 89 of the 106 currently recognised taxa from across Australia and 27 from outside of Australia. This includes 824 type specimens representing 45 currently recognised taxa and three synonymised taxa, comprising 43 holotypes, three syntypes and 779 paratypes. Of the paratypes, a total of 43 specimens have been gifted to other collections, disposed or could not be located and are considered lost. An annotated catalogue is provided for all agamid type material currently and previously maintained in the herpetological collection of the Western Australian Museum. KEYWORDS: type specimens, holotype, syntype, paratype, dragon lizard, nomenclature. INTRODUCTION Australia was named by John Edward Gray in 1825, The Agamidae, commonly referred to as dragon Clamydosaurus kingii Gray, 1825 [now Chlamydosaurus lizards, comprises over 480 taxa worldwide, occurring kingii (Gray, 1825)]. -

Reproductive Ecology of the Mountain Dragon, Rankin/A (Tympanocryptis) Diemensis (Reptilia: Squamata: Agamidae) in Tasmania
Papers and Proceedings ofthe Royal Society of Tasmania, Volume 139, 2005 23 REPRODUCTIVE ECOLOGY OF THE MOUNTAIN DRAGON, RANKIN/A (TYMPANOCRYPTIS) DIEMENSIS (REPTILIA: SQUAMATA: AGAMIDAE) IN TASMANIA by Jemina Stuart-Smith, Roy Swain and Andrew Welling (with three tables and two text-figures) Stuart-Smith, J., Swain, R. & Welling, A. 2005 (16:xii): Reproductive ecology of the Mountain Dragon, Rankinia (Tympanocryptis) diemensis (Reptilia: Squamata: Agamidae) in Tasmania. Papers and Proceedings of the Royal Society of Tasmania 139: 23-28. https://doi.org/10.26749/rstpp.139.23 ISSN 0080-4703. School of Zoology, University of Tasmania, Private Bag 5, Tasmania, 7001 Australia.(J.S-S.*, R.S., AW). *Author for correspondence. The mountain dragon, Rankinia (Tympanocryptis) diemensis (Gray, 1841), is the only member of the Agamidae in Tasmania. It occurs in some of the coldest regions occupied by any dragon in Australia, and is found in a variety of habitats ranging from coastal heath to alpine scrub. This paper examines the reproductive ecology of R. diemensis in the most southerly range of its distribution, providing baseline data on timing of reproductive events, reproductive cycles, nesting behaviour and ovipositioning, clutch characteristics and incubation conditions. Winter torpor lasts approximately seven months with males emerging in early September and spermatogenesis occurring from September-November. Females emerge later, with vitcllogenesis occurring from September-December. Gravid females may be found between October and January, but females are non-vitcllogenic from late December until the following season. The firstclutch is typically laid from October-December, with a variable clutch size (2-11 eggs). Females store sperm and a second clutch may be laid fiveweeks after the first. -

First Joint Scientific Webinar: Environmental Hazards
1st Joint Scientific Webinar: Environmental Hazards PRESIDENTS DR. BAHRAMI KAMANGAR BARZAN (UNIVERSITY OF KURDISTAN) DR. SAVENKOVA ELENA (RUDN) CO-PRESIDENTS DR. GHAHRAMANY LOGHMAN (UNIVERSITY OF KURDISTAN) FIRST JOINT SCIENTIFIC DR. POPKOVA ANNA (RUDN) WEBINAR: October 15, 2020 ENVIRONMENTAL HAZARDS Organizers: Faculty of Natural Resources - University of Kurdistan, IR Iran and Ecological Faculty - RUDN University, Russian Federation 1st Joint Scientific Webinar: Environmental Hazards 1 IMPACT OF GLOBAL CLIMATE CHANGE ON ECOSYSTEM FUNCTIONS OF AFRICAN COUNTRIES Kurbatova A. I. 1, Tarko A. M. 2 and Kozlova E. V.1 1. Peoples’ Friendship University of Russia, Moscow, Russia 2. Dorodnitsyn Computing Center, Russian Academy of Sciences, Moscow, Russia E-mail: [email protected] Abstract: Based on a global spatial mathematical model of the global carbon cycle in the biosphere, the change in environmental parameters caused by carbon dioxide emissions from fossil fuel combustion, deforestation, and erosion in African countries are calculated. The impact of deforestation and soil erosion due to inappropriate land use on climate change for African countries is calculated up to 2060. The calculations presented in the paper show that the power of regulatory functions of forest ecosystems in the period 2000–2020 is reduced on significant areas of the continental Africa due to their anthropo-genic degradation. Further change in the biosphere function of regulation of the carbon cycle depends on the ratio of opposite processes: on the one hand, intensification of the decomposition of organic matter in soils and the growth of CO2 emissions into the atmosphere from ecosystems; on the other hand, an increase in the productivity of ecosystems and their absorption of CO2 from the atmosphere. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

The Old Sheep Camp on Mount Majura
The Old Sheep Camp on Mount Majura Research on the history of the site and a management plan for its rehabilitation. By William Mudford Venturer Scout at Majura Mountain Scouts Research assisted by Waltraud Pix Co-ordinator of Friends of Mount Majura Park Care Group William Mudford’s Queen Scout Environment Project on Mount Majura Sheep Camp: Page 1 Introduction Mount Majura is located in the North of the Australian Capital Territory. A majority of Mount Majura is currently run as a Nature Reserve. Many parts of the reserve have infestations of non- indigenous plants, or weeds, because of prior land use and farming practices. One particular site, at the top of the Casuarina trail, on the saddle between Mount Majura and Mount Ainslie, known as the “Old Sheep Camp” is particularly infested with weeds. In my project I investigated the history of the site, the reasons for the weed infestation and the methods of rehabilitation the local park care group have available to them, and are utilising. This project seeks to answer the following questions about the site: 1. Who owned and managed the area and how was it used before it became a Nature Reserve? 2. What are the reasons for the heavy weed infestation on the site? 3. What processes and procedures can the local environment group use to rehabilitate the area? William Mudford’s Queen Scout Environment Project on Mount Majura Sheep Camp: Page 2 Contents Part 1 – The history of the area Page 4 Part 2 – The reasons for the infestation of non-indigenous flora at the Sheep Camp site. -

Assessment of Spatial and Temporal Variation in Surface Water Quality in Jerrabomberra Wetlands, Australian Capital Territory
Assessment of spatial and temporal variation in surface water quality in Jerrabomberra Wetlands, Australian Capital Territory Rahnum Tasnuva Nazmul A thesis in fulfillment of the requirements for the degree of Master of Philosophy School of Physical Environmental and Mathematical Sciences UNSW Canberra October 2016 THE UNIVERSITY OF NEW SOUTH WALES Thesis/Dissertation Sheet Surname or Family name: Nazmul First name: Rahnum Other name/s:Tasnuva Abbreviation for degree as given in the University calendar: MPhil School: School of Physical Environmental and Mathematical Faculty: UNSW Canberra Sciences Title: Assessment of spatial and temporal variation in surface water quality in Jerrabomberra Wetlands, Australian Capital Territory This Masters thesis aims to add to the knowledge of the spatio-temporal variation in surface water quality in Jerrabomberra Wetlands in order to provide information for managers as they seek to protect the values of the wetland, improve water quality and manage pollutants from the Fyshwick catchment. Located in the heart of Australian Capital Territory (ACT), Jerrabomberra Wetlands is a habitat for a variety of animals and plants. The Basin Priority Project (BPP), undertaken by the ACT and Commonwealth Governments to improve the quality of water flowing through the ACT includes this Fyshwick-Jerrabomberra catchment as a key site of mixed urban and agricultural land usage. Current study outcomes will add to the knowledge of the ACT wide water quality monitoring program. This project studied eight water quality parameters: water temperature, pH, turbidity, electrical conductivity, dissolved oxygen, total phosphorus and nitrate, and zinc using surface water samples collected from six locations at the south eastern corner of Jerrabomberra Wetlands on a weekly basis for four months in 2015. -

West Belconnen Strategic Assessment
WEST BELCONNEN PROJECT STRATEGIC ASSESSMENT Strategic Assessment Report FINAL March 2017 WEST BELCONNEN PROJECT STRATEGIC ASSESSMENT Strategic Assessment Report FINAL Prepared by Umwelt (Australia) Pty Limited on behalf of Riverview Projects Pty Ltd Project Director: Peter Cowper Project Manager: Amanda Mulherin Report No. 8062_R01_V8 Date: March 2017 Canberra 56 Bluebell Street PO Box 6135 O’Connor ACT 2602 Ph. 02 6262 9484 www.umwelt.com.au This report was prepared using Umwelt’s ISO 9001 certified Quality Management System. Executive Summary A Strategic Assessment between the Commonwealth The proposed urban development includes the Government and Riverview Projects commenced in provision of 11,500 dwellings, with associated services June 2014 under Part 10 of the Environment Protection and infrastructure (including the provision of sewer and Biodiversity Act 1999 (EPBC Act). The purpose of mains, an extension of Ginninderra Drive, and upgrade which was to seek approval for the proposed works to three existing arterial roads). It will extend development of a residential area and a conservation the existing Canberra town centre of Belconnen to corridor in west Belconnen (the Program). become the first cross border development between NSW and the ACT. A network of open space has also The Project Area for the Strategic Assessment been incorporated to link the WBCC to the residential straddles the Australian Capital Territory (ACT) and component and encourage an active lifestyle for the New South Wales (NSW) border; encompassing land community. west of the Canberra suburbs of Holt, Higgins, and Macgregor through to the Murrumbidgee River, and The aim of the WBCC is to protect the conservation between Stockdill Drive and Ginninderra Creek. -

South Jerrabomberra DCP 2015 Combined Table of Contents
South Jerrabomberra DCP 2015 Combined Table of Contents Date adopted by Council: 11 February 2015 Resolution number: PDRC006/15 Reference number: C1523204 Notification: 6 March 2015 South Jerrabomberra DCP – Combined Table of Contents Part 1 Preliminary 1.1 Introduction 1.2 Citation 1.3 Land Covered by this DCP 1.4 Date of Approval and Commencement of this DCP 1.5 Aims and Purpose of this DCP 1.6 Variation to this DCP 1.7 Relationship to Other Planning Instruments and Policies 1.8 How to Use this DCP 1.9 Pre-DA Process 1.10 Development Application Submission Requirements 1.11 Checklist for the Preparation of Neighbourhood Structure Plans 1.12 Checklist for Subdivision Applications Part 2 Context 2.1 Regional Planning Context Part 3 Master Plan 3.1 Master Planning for South Jerrabomberra 3.2 Master Plan Objectives 3.3 Master Plan Aims 3.4 Desired Future Character of Development Areas in South Jerrabomberra 3.5 Staging of Development in South Jerrabomberra 3.6 Neighbourhood Structure Plans 3.7 Neighbourhood Structure Plan Controls 3.8 Additional Controls for Buffer Land and Land on the Urban/Non-Urban Interface Part 4 Subdivision Controls 4.1 Introduction 4.2 Lot Size and Design 4.3 Lot Orientation 4.4 Lot Size and Layout 4.5 Bushfire Management 4.6 Additional Controls for Subdivision in a Buffer Area 2 Combined Table of Contents South Jerrabomberra DCP – Combined Table of Contents Part 5 Roads and Public Places 5.1 Introduction 5.2 Street Network 5.3 Local Sub-Arterial Road 5.4 Collector Road 5.5 Local Streets 5.6 Access Street 5.7 Edge