Diabetes and Pregnancy Program
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Childbirth Education Booklet
Childbirth Education Contents Normal Discomforts of Pregnancy 2 Call Your Doctor 4 Late Pregnancy 5 Labor: Stage 1 6 Labor: Stages 2 & 3 8 Breastfeeding in the Hospital 9 Breathing Techniques 10 Birth Plans 10 Labor Positions 11 How Your Partner and Doula Can Help 13 Packing for the Hospital 15 Normal Discomforts of Pregnancy Fatigue Backache • Listen to your body, take naps, get extra rest • Maintain correct posture • Do pelvic tilt exercises while standing and on hands Stuffy Nose and knees • Warm compresses to nose • While on all fours, crawl forwards and backwards, • Cool mist humidifier in your home or rock, and do pelvic tilts bedroom at night • When picking up something lift with your legs to protect your back Shortness of Breath • Don’t stay that way, slow down and catch your breath • For prolonged standing, elevate 1 foot on a step stool • Sleep propped up with pillows or in recliner • Receive back massages • Maintain correct posture Dribble Urine • Moderate intensity exercising (walking, stationary • Do Kegel exercises – at least 50 a day bike, swim, flexibility moves) (do 10 every time you wash your hands) • How can you tell if you are dribbling urine or your Heartburn/Nausea water broke? Call your OB or midwife, and remember • Don’t eat 3 big meals a day, eat 5-6 smaller meals this acronym: • Drink 8 cups of water a day COAT. C=color, O=odor, A=amount, T=time. • Have dry crackers/cereal to nibble on–keep in purse and at bedside • Don’t let stomach become empty • Eat well balanced diet–vitamin B may help to decrease • Avoid -

Gestational Diabetes Insipidus, HELLP Syndrome and Eclampsia in a Twin Pregnancy: a Case Report
Journal of Perinatology (2010) 30, 144–145 r 2010 Nature Publishing Group All rights reserved. 0743-8346/10 $32 www.nature.com/jp PERINATAL/NEONATAL CASE PRESENTATION Gestational diabetes insipidus, HELLP syndrome and eclampsia in a twin pregnancy: a case report JL Woelk, RA Dombroski and PR Brezina Department of Obstetrics and Gynecology, East Carolina University, Greenville, NC, USA alanine aminotransferase, 65 U lÀ1; and lactate dehydrogenase, We report a case of eclampsia in a twin pregnancy complicated by HELLP 390 U lÀ1. A 24-h urine collection was begun to quantify proteinuria. syndrome and diabetes insipidus. This confluence of disease processes During the first night of hospitalization, the patient developed suggests that a modification of common magnesium sulfate treatment marked polyuria and polydipsia. Urine output increased to 1500 cc hÀ1 protocols may be appropriate in a certain subset of patients. by the early morning totaling 12 l for the entire night. Repeat Journal of Perinatology (2010) 30, 144–145; doi:10.1038/jp.2009.115 electrolytes were unchanged. The endocrinology service was then Keywords: diabetes insipidus ; eclampsia ; HELLP syndrome ; twin consulted for the management of presumptive DI. Therapy with the pregnancy ; magnesium sulfate administration of dDAVP (1-deamino-8-D-arginine vasopressin) orally twice daily was initiated. Serum osmolality was increased at 296 mOsm kgÀ1, and urine osmolality was decreased to 71 mOsm kgÀ1 Introduction with a specific gravity of 1.000. The 24-h urine results showed a total The association between diabetes insipidus (DI) and liver protein of 780 mg. The patient denied the previously reported dysfunction in a preeclamptic patient has been established in headaches, blurry vision and right upper quadrant pain, and her blood multiple case reports.1 This case is an important example that pressures remained 140 to 155 mmHg (systolic) and 80 to 95 mmHg illustrates how the pathophysiology of DI and the pharmacokinetics (diastolic), consistent with a diagnosis of mild preeclampsia. -

Combining a Glucagon-Like Peptide-1 Receptor Agonist with Basal Insulin: the Why and How
Combining a Glucagon-like Peptide-1 Receptor Agonist with Basal Insulin: The Why and How Case Study Mary is a 61 year-old female diagnosed with type 2 diabetes mellitus (T2DM) 8 years ago. She was initially managed with the combination of lifestyle modification and metformin. Since that time she was treated with a sulfonylurea, but it was discontinued due to symptomatic hypoglycemia. She was also treated with pioglitazone, but significant fluid retention led to it discontinuation. A year-and-a- half ago, basal insulin was added to her lifestyle and metformin management. She now administers 52 units (0.62 units/kg) once daily at bedtime. Since starting basal insulin, she has experienced 3 episodes of mild hypoglycemia. Since her diagnosis, Mary’s HbA1c has never been <7.0%; her current HbA1c is 7.9%. Over the past month, her fasting plasma glucose (FPG) has ranged from 103 mg/dL to 136 mg/dL and her postprandial glucose (PPG) from 164 mg/dL to 213 mg/dL. She has gained 2.6 kg since starting basal insulin and her body mass index is now 31 kg/m2. Her blood pressure is 134/82 mmHg. She experiences occasional tingling in her feet. Eye examination reveals grade 1 retinopathy. Current medications are: metformin 1000mg twice daily, basal insulin 52 units once daily at bedtime, and hydrochlorothiazide 25 mg once daily. Her family physician notes that Mary’s FPG is reasonably well-controlled, yet her HbA1c and PPG remain elevated. He is also concerned about her episodes of hypoglycemia and weight gain and the evidence indicating microvascular damage. -

The Effects of Alcohol in Newborns Efeitos Do Álcool No Recém-Nascido
REVIEW The effects of alcohol in newborns Efeitos do álcool no recém-nascido Maria dos Anjos Mesquita* ABSTRACT alcoólicas leva a prejuízos individuais, para a sua família e para The purpose of this article was to present a review of the effects toda a sociedade. Apesar disso, a dificuldade do seu diagnóstico e of alcohol consumption by pregnant mothers on their newborn. a inexperiência dos profissionais de saúde faz com que o espectro Definitions, prevalence, pathophysiology, clinical features, diagnostic dessas lesões seja pouco lembrado e até desconhecido. As lesões criteria, follow-up, treatment and prevention were discussed. A causadas pela ação do álcool no concepto são totalmente prevenidas search was performed in Medline, LILACS, and SciELO databases se a gestante não consumir bebidas alcoólicas durante a gestação. using the following terms: “fetus”, “newborn”, “pregnant woman”, Assim, é fundamental a detecção das mulheres consumidoras “alcohol”, “alcoholism”, “fetal alcohol syndrome”, and “alcohol- de álcool durante a gravidez e o desenvolvimento de programas related disorders”. Portuguese and English articles published from específicos de alerta sobre as consequências do álcool durante a 2000 to 2009 were reviewed. The effects of alcohol consumed by gestação e amamentação. pregnant women on newborns are extremely serious and occur frequently; it is a major issue in Public Health worldwide. Fetal alcohol Descritores: Bebidas alcoólicas/efeitos adversos; Feto; Recém-nascido; spectrum disorders cause harm to individuals, their families, and the Síndrome alcoólica fetal; Transtornos relacionados ao uso de álcool entire society. Nevertheless, diagnostic difficulties and inexperience of healthcare professionals result in such damage, being remembered rarely or even remaining uncovered. -

Care During Pregnancy and Delivery ACCESSIBLE, QUALITY HEALTH CARE DURING PREGNANCY and DELIVERY
Care during Pregnancy and Delivery ACCESSIBLE, QUALITY HEALTH CARE DURING PREGNANCY AND DELIVERY Why It’s Important Having a healthy pregnancy and access to quality birth facilities are the best ways to promote a healthy birth and have a thriving newborn. Getting early and regular prenatal care is vital. Prenatal care is the health care that women receive during their entire pregnancy. Prenatal care is more than doctor’s visits and ultrasounds; it is an opportunity to improve the overall well-being and health of the mom which directly affects the health of her baby. Prenatal visits give parents a chance to ask questions, discuss concerns, treat complications in a timely manner, and ensure that mom and baby are safe during pregnancy and delivery. Receiving quality prenatal care can have positive effects long after birth for both the mother and baby. When it is time for the mother to give birth, having access to safe, high quality birth facilities is critical. Early prenatal care, starting in the 1st trimester, is crucial to the health of mothers and babies. But more important than just initiating early prenatal care is receiving adequate prenatal care, having the appropriate number of prenatal care visits at the appropriate intervals throughout the pregnancy. Babies of mothers who do not get prenatal care are three times more likely to be born low birth weight and five times more likely to die than those born to mothers who do get care.1 In 2017 in Minnesota, only 77.1 percent of women received prenatal care within their first trimester of pregnancy. -

PLANNED OUT-OF-HOSPITAL BIRTH Approved 11/12/15
HEALTH EVIDENCE REVIEW COMMISSION (HERC) COVERAGE GUIDANCE: PLANNED OUT-OF-HOSPITAL BIRTH Approved 11/12/15 HERC COVERAGE GUIDANCE Planned out-of-hospital (OOH) birth is recommended for coverage for women who do not have high- risk coverage exclusion criteria as outlined below (weak recommendation). This coverage recommendation is based on the performance of appropriate risk assessments1 and the OOH birth attendant’s compliance with the consultation and transfer criteria as outlined below. Planned OOH birth is not recommended for coverage for women who have high risk coverage exclusion criteria as outlined below, or when appropriate risk assessments are not performed, or where the attendant does not comply with the consultation and transfer criteria as outlined below (strong recommendation). High-risk coverage exclusion criteria: Complications in a previous pregnancy: Maternal surgical history Cesarean section or other hysterotomy Uterine rupture Retained placenta requiring surgical removal Fourth-degree laceration without satisfactory functional recovery Maternal medical history Pre-eclampsia requiring preterm birth Eclampsia HELLP syndrome Fetal Unexplained stillbirth/neonatal death or previous death related to intrapartum difficulty Baby with neonatal encephalopathy Placental abruption with adverse outcome Complications of current pregnancy: Maternal Induction of labor Prelabor rupture of membranes > 24 hours 1 Pre-existing chronic hypertension; Pregnancy-induced hypertension with diastolic blood pressure greater than -
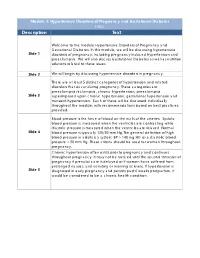
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text Welcome to the module Hypertensive Disorders of Pregnancy and Gestational Diabetes. In this module, we will be discussing hypertensive Slide 1 disorders of pregnancy, including pregnancy induced hypertension and preeclampsia. We will also discuss Gestational Diabetes as well as nutrition solutions related to these issues Slide 2 We will begin by discussing hypertensive disorders in pregnancy. There are at least 5 distinct categories of hypertension and related disorders that occur during pregnancy. These categories are: preeclampsia/eclampsia, chronic hypertension, preeclampsia Slide 3 superimposed upon chronic hypertension, gestational hypertension and transient hypertension. Each of these will be discussed individually throughout the module, with recommendations based on best practices provided. Blood pressure is the force of blood on the walls of the arteries. Systolic blood pressure is measured when the ventricles are contracting while diastolic pressure is measured when the ventricles are relaxed. Normal Slide 4 blood pressure is typically 120/80 mm Hg.The general definition of high blood pressure in adults is a systolic BP > 140 mg HG or a diastolic blood pressure > 90 mm Hg. These criteria should be used for women throughout pregnancy. Chronic hypertension often exists prior to pregnancy and continues throughout pregnancy. It may not be noticed until the second trimester of pregnancy if prenatal care is delayed or if women have suffered from prolonged nausea and vomiting or morning sickness. If hypertension is Slide 5 diagnosed in early pregnancy and persists past 6 weeks postpartum, it would be considered to be a chronic health condition. -

Role of Maternal Age and Pregnancy History in Risk of Miscarriage
RESEARCH Role of maternal age and pregnancy history in risk of BMJ: first published as 10.1136/bmj.l869 on 20 March 2019. Downloaded from miscarriage: prospective register based study Maria C Magnus,1,2,3 Allen J Wilcox,1,4 Nils-Halvdan Morken,1,5,6 Clarice R Weinberg,7 Siri E Håberg1 1Centre for Fertility and Health, ABSTRACT Miscarriage and other pregnancy complications might Norwegian Institute of Public OBJECTIVES share underlying causes, which could be biological Health, PO Box 222 Skøyen, To estimate the burden of miscarriage in the conditions or unmeasured common risk factors. N-0213 Oslo, Norway Norwegian population and to evaluate the 2MRC Integrative Epidemiology associations with maternal age and pregnancy history. Unit at the University of Bristol, Introduction Bristol, UK DESIGN 3 Miscarriage is a common outcome of pregnancy, Department of Population Prospective register based study. Health Sciences, Bristol Medical with most studies reporting 12% to 15% loss among School, Bristol, UK SETTING recognised pregnancies by 20 weeks of gestation.1-4 4Epidemiology Branch, National Medical Birth Register of Norway, the Norwegian Quantifying the full burden of miscarriage is Institute of Environmental Patient Register, and the induced abortion register. challenging because rates of pregnancy loss are Health Sciences, Durham, NC, USA PARTICIPANTS high around the time that pregnancies are clinically 5Department of Clinical Science, All Norwegian women that were pregnant between recognised. As a result, the total rate of recognised University of Bergen, Bergen, 2009-13. loss is sensitive to how early women recognise their Norway pregnancies. There are also differences across countries 6 MAIN OUTCOME MEASURE Department of Obstetrics and studies in distinguishing between miscarriage and and Gynecology, Haukeland Risk of miscarriage according to the woman’s age and University Hospital, Bergen, pregnancy history estimated by logistic regression. -

Type 1 Diabetes and Pregnancy
Type 1 diabetes and pregnancy Information for patients 1 2 Contents How will diabetes affect my pregnancy? 4 How will diabetes affect my baby? 5 My antenatal care 6 Hypoglycaemia 9 Planning for your birth 12 After your baby is born 15 Nutrition and related issues 17 Diet and lifestyle Controlling your blood glucose levels with diet Fluid intake Fat in foods Calcium Iron Folic acid and diabetes Diabetic products Symptoms in pregnancy How much weight should I expect to put on? Physical activity Suitable snacks Additional information Breastfeeding and diabetes 31 What happens once my baby is born? 34 For the future 37 References and useful links 39 3 This booklet aims to tell you what care to expect during and after your pregnancy when you have diabetes. It will not answer every question you may have, so please write any concerns down and ask your midwife or doctor. We run a joint antenatal and diabetes clinic at Barnet Hospital and the Royal Free Hospital. We have a dedicated multidisciplinary team who will review you on a regular basis to provide advice and support throughout your pregnancy. The team consists of a consultant endocrinologist, obstetrician, diabetes specialist midwife, diabetes specialist nurse and a diabetes specialist dietitian. How will diabetes affect my pregnancy? With good pre-conception care and good diabetes control there is every chance of a successful pregnancy. As soon as your pregnancy is confirmed, you should attend the joint diabetes antenatal clinic. Here you will receive support and advice to enable you to obtain and maintain good control of your diabetes as early in your pregnancy as possible. -

Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study
life Article Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study Ho Yeon Kim 1, Geum Joon Cho 1,*, So Yeon Kim 1, Kyu-Min Lee 2, Ki Hoon Ahn 1 , Sung Won Han 2, Soon-Cheol Hong 1, Hyun Mee Ryu 3, Min-Jeong Oh 1, Hai-Joong Kim 1 and Seung Chul Kim 4,* 1 Department of Obstetrics and Gynecology, Korea University College of Medicine, 27 Inchonro, Seongbuk-gu, Seoul 02841, Korea; [email protected] (H.Y.K.); [email protected] (S.Y.K.); [email protected] (K.H.A.); [email protected] (S.-C.H.); [email protected] (M.-J.O.); [email protected] (H.-J.K.) 2 School of Industrial Management Engineering, Korea University, 145 Anam-ro, Anam-dong, Seongbuk-gu, Seoul 02841, Korea; [email protected] (K.-M.L.); [email protected] (S.W.H.) 3 Department of Obstetrics and Gynecology, CHA Bungdang Medical Center, CHA University School of Medicine, 59 Yatap-ro, Bundang-gu, Seongnam-si, Gyeonggi-do 13496, Korea; [email protected] 4 Department of Obstetrics and Gynecology, Pusan National University School of Medicine, 2 Busandaehak-ro 63beon-gil, Jangjeon 2(i)-dong, Geumjeong-gu, Busan 46241, Korea * Correspondence: [email protected] (G.J.C.); [email protected] (S.C.K.) Abstract: Hyperemesis gravidarum is known to be associated with poor perinatal outcomes. This study aimed to identify pre-pregnancy risk factors for hospital admission in women with hyperemesis gravidarum. We enrolled women who had delivered between 1 January 2013 and 31 December 2015, and had undergone a national health screening examination through the National Health Insurance Corporation 1–2 years before their first delivery. -

What You Need to Know About Morning Sickness What Is Morning Sickness? Morning Sickness Is Nausea And/Or Vomiting That Many Pregnant Women Experience
What You Need to Know About Morning Sickness What is morning sickness? Morning sickness is nausea and/or vomiting that many pregnant women experience. The term “morning sickness” is common, but it’s not correct, because many women have nausea and vomiting all day. The most important thing to know about nausea you may experience during your pregnancy is it’s normal. According to the American Pregnancy Association, more than 50% of pregnant women have nausea and/or vomiting. Although it’s most common during the first trimester, it’s possible to feel sick throughout the entire nine months of your pregnancy. For some women, feeling nauseous and/or throwing up are among the first symptoms of pregnancy. Most women start having nausea and/or vomiting around the sixth week of their first trimester. And some women notice their symptoms disappear around the 12th week of pregnancy or their second trimester. In general, nausea when pregnant isn’t harmful to you or the baby. However, if you can’t keep water or food down for long periods, then it can be dangerous, and you should talk to your provider about it. Common symptoms • Nausea • Vomiting • Feeling sick • Not being able to handle specific odors or foods Extreme morning sickness: Hyperemesis gravidarum Estimates are that 3% of pregnant women have hyperemesis gravidarum. This extreme nausea, vomiting and weight loss during pregnancy can be harmful to you and the baby, so you should talk to your doctor right away. If you’re not able to keep food or water down, then you could become malnourished and dehydrated. -

Alcohol, Breastfeeding, and Development at 18 Months
Alcohol, Breastfeeding, and Development at 18 Months Ruth E. Little, ScD*; Kate Northstone, MSc‡; Jean Golding, PhD, ScD‡; and the ALSPAC Study Team‡ ABSTRACT. Objective. We aimed to replicate a pre- literature search, we found only 1 systematic study1 vious study of 1-year-olds that reported a deficit in motor that measured drinking during lactation and subse- development associated with moderate alcohol use dur- quent child development. The authors reported a ing lactation, using a different but comparable popula- decrease in the motor development of infants at 1 tion. year of age, as measured by the Bayley Scales,2 when Methodology. The mental development of 915 18- the lactating mother had as little as 1 drink daily in month-old toddlers from a random sample of a longitu- dinal population-based study in the United Kingdom the first 3 months after delivery. This result was was measured using the Griffiths Developmental Scales. independent of drinking during pregnancy. Frequent self-administered questionnaires during and Lactation is the preferred method of early infant after pregnancy provided maternal data. The dose of feeding, and drinking alcohol is a common social alcohol available to the lactating infant was obtained by custom in most of the western world. Knowing the multiplying the alcohol intake of the mother by the pro- effect on the infant when lactation and alcohol drink- portion of breast milk in the infant’s diet. We compared ing are combined is of high importance, especially in this dose with the Griffiths Scales of Mental Develop- view of the previous report of its adverse effects.