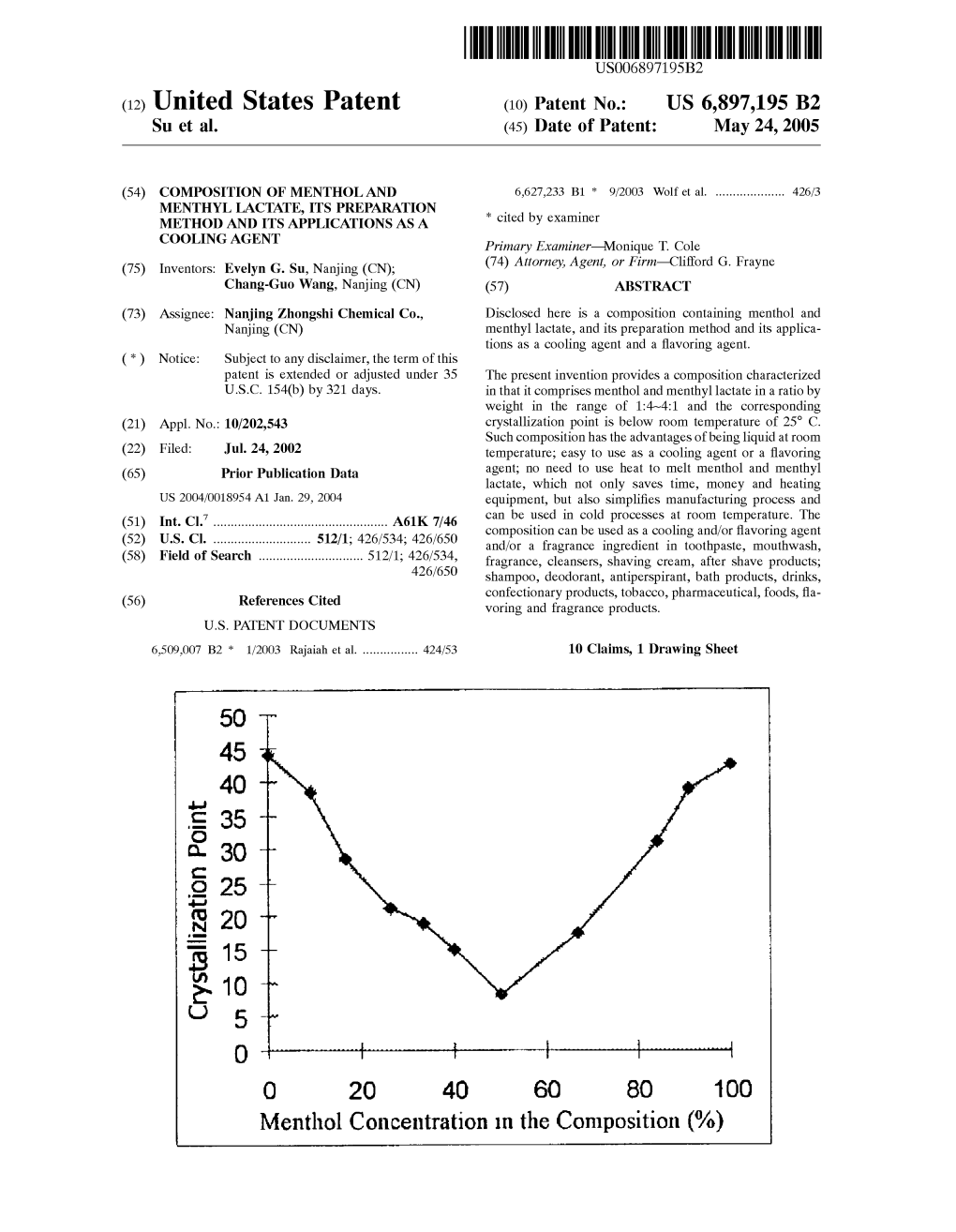
Menthol Concentration in the Composition (%) U.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Your Reliable Partner DELIVERING SAFE and QUALITATIVE PRODUCTS for HIGH PERFORMANCE SOLUTIONS
Your reliable partner DELIVERING SAFE AND QUALITATIVE PRODUCTS FOR HIGH PERFORMANCE SOLUTIONS corbion.com/biochemicals Why Corbion? Advanced technology and R&D At Corbion, we engage in ongoing R&D efforts to improve performance and sustainability of our products and processes. Consistent high quality Corbion has mastered the production technology to make high purity, high performance lactic acid, derivatives and lactides at industrial scale. Corbion factory in The Netherlands 100 100 years of experience Corbion has 100 years of experience in sales, application development and industrial scale production. Corbion is the global market leader in lactic acid, derivatives and lactides. Your reliable Global presence With 10 production facilities and sales offices on every partner continent, we are always close by to help you with your application development. All our products are available at an industrial scale Innovation and Application Our innovation and Application centers are focused on your Safer and more friendly for our planet challenges of tomorrow. Our technical team of chemist, analytical Corbion produces high quality lactic acid and derivatives and application technologist are available to provide you with using a biochemical fermentation process by efficient customized solutions. conversion of sugars. Corbion products are regarded as safe, offering a good alternative to traditional products that Deliveries have become under increased regulatory pressure. Corbion works on continuous improvement of its delivery times and reliability. Working to improve consistency within the supply chain is making the network more responsive while streamlining operations. Prevention Corbion deploys prevention activities in our plants and throughout our supply chain in order to avoid the occurrence of incidents. -

United States Patent Office Patented Oct
2,909,466 United States Patent Office Patented Oct. 20, 1959 1 2 tempts have always failed because the solutions were not 2,909,466 stable or, if sufficiently stable, they contained an insuffi cient amount of oxytetracycline salt, or because the sol STABLE SOLUTIONS OF OXYTETRACYCLINE vent which did produce stable solutions raised consider SAILS able doubts with respect to its pharmacological properties. Horst Neumann, Bingen, and Paul Viehmann and Hans It is an object of the present invention to provide stable Hugo Hibner, Engelheim, Germany, assignors, by Solutions of oxytetracycline salts which contain a suffi mesne assignments, to Chas. Pfizer & Co., Inc., Brook cient amount of the antibotic for effective therapeutic ad lyn, N.Y., a corporation of Delaware ministration. No Drawing. Application May 23, 1957 10 Another object of the present invention is to provide a Serial No. 661,033 stable solution of oxytetracycline salts wherein the sol vent has no objectionable pharmacological properties. Claims priority, application Germany May 29, 1956 Other objects and advantages of the present invention 9 Claims. (Cl. 167-65) will become apparent as the description proceeds. 5 The above-indicated objects and advantages are This invention relates to stable solutions of oxytetra achieved if a lower alkyl alcohol ester of lactic acid is -cycline salts, and more particularly to solutions of oxy used as the solvent for the oxytetracycline salt. tetracycline salts in lower alkyl alcohol esters of lactic The superior stability of oxytetracycline salt -

Purification of Lactic Acid
PURIFICATION OF LACTIC ACID by SIDNEY HSIN-HUAI CHOW B. S., Taiwan Provincial Cheng Kung University, 1957 A MASTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Chemical Engineering KANSAS STATE UNIVERSITY Manhattan, Kansas 1962 11 TABLE OF CONTENTS INTRODUCTION 1 Properties of Lactic Acid 1 Preparation of Lactic Acid 3 Utilization of Lactic Acid 13 Purification of Lactic Acid 14 Purpose of this Research 23 DESCRIPTION OF THE PROCESS 24 Materials 24 Equipment 25 Experimental Procedure 26 Analytical Methods . 27 EXPERIMENTAL RESULTS 29 Material Losses 29 Yields 29 Effect of Feed Composition 32 Effect of Time of Run 32 Effect of Concentration of Catalyst 33 Effect of Temperature 33 Purity of the Products 33 DISCUSSION OF RESULTS 34 RECOMMENDATIONS 35 ACKNOWLEDGMENTS 38 LITERATURE CITED 39 APPENDIX 41 INTRODUCTION Properties of Lactic Acid OH Lactic acid is alphahydroxy propionic acid, CH3---C---COOH. Because of the asymmetry of the alpha carbon atom, this acid exists in two modifi- cations, a dextro acid and a levo acid. COOH COOH HO OH CH3 CH3 Dextro -Lactic Acid Levo-Lactic Acid The commercial acid is a mixture of the two forms, usually in equal proportions; and it is, therefore, inactive with respect to the rotation of the plane of polarized light. Lactic acid is both an alcohol and an acid; and, therefore, its molecules can form esters with one another. In water solutions containing less than 20 percent of lactic acid, the acid is in the simple monomeric form; but solutions of greater concentration contain some esters involving two or more of the simple molecules. -

Identification of Novel Simulants for Toxic Industrial Chemicals
International Journal of Environmental Research and Public Health Review Identification of Novel Simulants for Toxic Industrial Chemicals and Chemical Warfare Agents for Human Decontamination Studies: A Systematic Review and Categorisation of Physicochemical Characteristics Thomas James * , Samuel Collins and Tim Marczylo Centre for Radiation, Chemicals and Environmental Hazards (CRCE), Public Health England, Chilton OX11 0RQ, UK; [email protected] (S.C.); [email protected] (T.M.) * Correspondence: [email protected] Abstract: Chemical simulants have long been used in human trials of mass decontamination to determine the efficacy of decontamination interventions against more toxic agents. Until now, reliance has mostly been on individual chemicals as surrogates to specific agents (e.g., methyl salicylate for sulphur mustard). A literature review was conducted to identify chemicals that had been previously tested on human volunteers and that represent diverse physicochemical characteristics in order to create a repository for chemical simulants. Of the 171 unique chemicals identified, 78 were discounted for the risk they could pose to human volunteers, 39 were deemed suitable for use, and a further 54 were considered to be possible simulants but would require further research. Suitable simulants Citation: James, T.; Collins, S.; included both solid and liquid chemicals spanning a wide range of physicochemical properties Marczylo, T. Identification of Novel including molecular weight, octanol/water partition coefficient, vapour pressure, and solubility. This Simulants for Toxic Industrial review identifies an array of potential simulants suitable for use in human volunteer decontamination Chemicals and Chemical Warfare studies and is of relevance to future studies on systemic absorption and surface decontamination. -

DECOS and SCG Basis for an Occupational Standard. Lactate Esters
1999:9 DECOS and SCG Basis for an Occupational Standard Lactate esters Per Lundberg arbete och hälsa vetenskaplig skriftserie ISBN 91–7045–519–8 ISSN 0346–7821 http://www.niwl.se/ah/ National Institute for Working Life National Institute for Working Life The National Institute for Working Life is Sweden’s national centre for work life research, development and training. The labour market, occupational safety and health, and work organisation are our main fields of activity. The creation and use of knowledge through learning, information and documentation are important to the Institute, as is international co- operation. The Institute is collaborating with interested parties in various development projects. The areas in which the Institute is active include: • labour market and labour law, • work organisation, • musculoskeletal disorders, • chemical substances and allergens, noise and electromagnetic fields, • the psychosocial problems and strain-related disorders in modern working life. ARBETE OCH HÄLSA Editor-in-Chief: Staffan Marklund Co-Editors: Mikael Bergenheim, Anders Kjellberg, Birgitta Meding, Gunnar Rosén and Ewa Wigaeus Hjelm © National Institute for Working Life & authors 1999 National Institute for Working Life, 171 84 Solna, Sweden ISBN 91–7045–519–8 ISSN 0346-7821 http://www.niwl.se/ah/ Printed at CM Gruppen Preface An agreement has been signed by the Dutch Expert Committee on Occupational Standards (DECOS) of the Dutch Health Council and the Swedish Criteria Group for Occupational Standards (SCG) of the Swedish National Institute for Working Life. The purpose of the agreement is to write joint scientific criteria documents for occupational exposure limits. The numerical limits will be developed separately by The Netherlands and Sweden according to their different national policies. -

Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002
Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002 James S. Chickosa… Department of Chemistry, University of Missouri-St. Louis, St. Louis, Missouri 63121 William E. Acree, Jr.b… Department of Chemistry, University of North Texas, Denton, Texas 76203 ͑Received 17 June 2002; accepted 17 October 2002; published 21 April 2003͒ A compendium of vaporization enthalpies published within the period 1910–2002 is reported. A brief review of temperature adjustments of vaporization enthalpies from temperature of measurement to the standard reference temperature, 298.15 K, is included as are recently suggested reference materials. Vaporization enthalpies are included for organic, organo-metallic, and a few inorganic compounds. This compendium is the third in a series focusing on phase change enthalpies. Previous compendia focused on fusion and sublimation enthalpies. Sufficient data are presently available for many compounds that thermodynamic cycles can be constructed to evaluate the reliability of the measure- ments. A protocol for doing so is described. © 2003 American Institute of Physics. ͓DOI: 10.1063/1.1529214͔ Key words: compendium; enthalpies of condensation; evaporation; organic compounds; vaporization enthalpy. Contents inorganic compounds, 1880–2002. ............. 820 1. Introduction................................ 519 8. References to Tables 6 and 7.................. 853 2. Reference Materials for Vaporization Enthalpy Measurements.............................. 520 List of Figures 3. Heat Capacity Adjustments. ................. 520 1. A thermodynamic cycle for adjusting vaporization ϭ 4. Group Additivity Values for C (298.15 K) enthalpies to T 298.15 K.................... 521 pl 2. A hypothetical molecule illustrating the different Estimations................................ 521 hydrocarbon groups in estimating C ........... 523 5. A Thermochemical Cycle: Sublimation, p Vaporization, and Fusion Enthalpies........... -

Catalytic Conversion of Glycerol to Value-Added Chemicals in Alcohol
Fuel Processing Technology 140 (2015) 148–155 Contents lists available at ScienceDirect Fuel Processing Technology journal homepage: www.elsevier.com/locate/fuproc Catalytic conversion of glycerol to value-added chemicals in alcohol Shoujie Ren, X. Philip Ye ⁎ Department of Biosystems Engineering and Soil Science, The University of Tennessee, 2506 E. J. Chapman Drive, Knoxville, TN 37996, USA article info abstract Article history: With the aim to directly use the mixture of glycerol and methanol from the biodiesel production for value-added Received 19 June 2015 chemical production, catalytic conversion of refined glycerol to lactic acid (LA) and propylene glycol (PG) using Received in revised form 3 September 2015 mixed solid catalysts of CaO and CuO in methanol medium was first investigated. At the optimum condition, the Accepted 7 September 2015 yields of LA and PG achieved were 46 mol% and 35 mol%, respectively. For recycling the catalysts, a combined pro- Available online 15 September 2015 cess of glycerol conversion to alkyl lactate was further investigated. Using this integrated process, 45 mol% methyl Keywords: lactate yield and 28 mol% PG yield were achieved in methanol medium, and 45 mol% ethyl lactate and 18 mol% PG Glycerol yield were achieved in ethanol medium. The test for crude glycerol conversion showed that the impurities had Alkyl lactate slightly negative effects on glycerol conversion and product yield in methanol medium but no obvious effect in Lactic acid ethanol medium. Similar glycerol conversion and product yield were obtained when the mixture of glycerol, Methanol methanol, and CaO from biodiesel production were directly used as starting material. -

United States Patent Office Patented July 31, 1973 2 Good Properties Such As Water-Proofness, Adhesiveness 3,749,769 and Luster to Nitrocellulose Lacquers
3,749,769 United States Patent Office Patented July 31, 1973 2 good properties such as water-proofness, adhesiveness 3,749,769 and luster to nitrocellulose lacquers. NAIL LACQUER COMPOSITIONS AND PROCESS FOR THE PREPARATION THEREOF DETALED DESCRIPTION Iwakichi Sugiyama, Narashino, and Haruki Tomozuka, The oligomers and co-oligomers of acrylic esters used Tokyo, Japan, assignors to Matsumoto Chemical indus try Co., Ltd., Ichikawa-shi, Chiba-ken, Japan in the invention can be prepared by polymerizing acrylic No Drawing. Continuation of abandoned application Ser. ester monomers in the medium of a great quantity of the No. 753,745, Aug. 19, 1968. This application Jan. 22, solvent having a relatively large chain-transfer constant. 1971, Ser. No. 108,971 Such solvents, include, for instance, methyl isobutyl Claims priority, application Japan, Feb. 1, 1968, O ketone, methyl ethyl ketone, isobutyl alcohol, isopropyl 43/5,832 alcohol, sec, butyl alcohol, and toluene. These solvents int, C. A61k 7/04 are used in amounts of 4-20 moles, preferably 6-15 U.S. C. 424-6 5 Clains moles, per mole of the acrylic ester monomers. As mentioned above, the oligomers or co-oligomers ABSTRACT OF THE DISCLOSURE 5 used in the invention can be synthesized with the use of A nail lacquer having a good water-proofness, ad generally known solvents having a large chain-transfer hesiveness and luster is obtained by polymerizing acrylic constant. But it is necessary to make the molar ratio of monomers in a solvent having a relatively large chain solvent to monomer considerably large, and yet the yield transfer constant to form oligomers or co-oligomers, of the intended oligomer is very low. -

Sustainable Fabrication of Organic Solvent Nanofiltration Membranes
membranes Review Sustainable Fabrication of Organic Solvent Nanofiltration Membranes Hai Yen Nguyen Thi 1, Bao Tran Duy Nguyen 1 and Jeong F. Kim 1,2,* 1 Department of Energy and Chemical Engineering, Incheon National University, Incheon 22012, Korea; [email protected] (H.Y.N.T.); [email protected] (B.T.D.N.) 2 Innovation Center for Chemical Engineering, Incheon National University, Incheon 22012, Korea * Correspondence: [email protected] Abstract: Organic solvent nanofiltration (OSN) has been considered as one of the key technologies to improve the sustainability of separation processes. Recently, apart from enhancing the membrane performance, greener fabricate on of OSN membranes has been set as a strategic objective. Consider- able efforts have been made aiming to improve the sustainability in membrane fabrication, such as replacing membrane materials with biodegradable alternatives, substituting toxic solvents with greener solvents, and minimizing waste generation with material recycling. In addition, new promis- ing fabrication and post-modification methods of solvent-stable membranes have been developed exploiting the concept of interpenetrating polymer networks, spray coating, and facile interfacial polymerization. This review compiles the recent progress and advances for sustainable fabrication in the field of polymeric OSN membranes. Keywords: sustainability; organic solvent nanofiltration; green solvents; environmental-friendly polymers; bio-based polymers 1. Introduction With escalating environmental concerns, the concept of sustainability has become more Citation: Nguyen Thi, H.Y.; Nguyen, important. The environmental regulations are getting even more stringent, and industries B.T.D.; Kim, J.F. Sustainable Fabrica- tion of Organic Solvent Nanofiltration are widely implementing membrane technology to improve their process sustainability. -

The Occurrence of Propyl Lactate in Chinese Baijius (Chinese Liquors) Detected by Direct Injection Coupled with Gas Chromatography-Mass Spectrometry
Molecules 2015, 20, 19002-19013; doi:10.3390/molecules201019002 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article The Occurrence of Propyl Lactate in Chinese Baijius (Chinese Liquors) Detected by Direct Injection Coupled with Gas Chromatography-Mass Spectrometry Jihong Wu 1,2, Yang Zheng 1,2, Baoguo Sun 1,2,3, Xiaotao Sun 1,2, Jiyuan Sun 1,2, Fuping Zheng 1,2 and Mingquan Huang 1,2,3,* 1 School of Food and Chemical Engineering, Beijing Technology and Business University, Beijing 100048, China; E-Mails: [email protected] (J.W.); [email protected] (Y.Z.); [email protected] (B.S.); [email protected] (X.S.); [email protected] (J.S.); [email protected] (F.Z.) 2 Beijing Key Laboratory of Flavor Chemistry, Beijing Technology and Business University, Beijing 100048, China 3 Beijing Innovation Centre of Food Nutrition and Human Health, Beijing 100048, China * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +86-10-6898-5382. Academic Editor: Luca Forti Received: 1 September 2015 / Accepted: 13 October 2015 / Published: 19 October 2015 Abstract: As one of the oldest distillates in the world, flavor compounds of Chinese Baijiu (Chinese liquor) were extremely complex. Propyl lactate was firstly detected by direct injection and gas chromatography-mass spectrometry (GC-MS) in 72 Chinese Baijius. The objectives were to detect the contents of propyl lactate and evaluate its contribution to the aroma of Chinese Baijiu based on odor activity values (OAVs). The levels of propyl lactate in these distillates were determined by internal standard method and selective ion monitoring (SIM), which ranged from 0.050 to 1.900 mg·L−1 under investigation. -

Kinetics of Alkyl Lactate Formation from the Alcoholysis of Poly(Lactic Acid)
processes Article Kinetics of Alkyl Lactate Formation from the Alcoholysis of Poly(Lactic Acid) Fabio M. Lamberti 1, Luis A. Román-Ramírez 1, Paul Mckeown 2 , Matthew D. Jones 2 and Joseph Wood 1,* 1 School of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK; [email protected] (F.M.L.); [email protected] (L.A.R.-R.) 2 Department of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK; [email protected] (P.M.); [email protected] (M.D.J.) * Correspondence: [email protected] Received: 8 June 2020; Accepted: 23 June 2020; Published: 24 June 2020 Abstract: Alkyl lactates are green solvents that are successfully employed in several industries such as pharmaceutical, food and agricultural. They are considered prospective renewable substitutes for petroleum-derived solvents and the opportunity exists to obtain these valuable chemicals from the chemical recycling of waste poly(lactic acid). Alkyl lactates (ethyl lactate, propyl lactate and butyl lactate) were obtained from the catalysed alcoholysis reaction of poly(lactic acid) with the corresponding linear alcohol. Reactions were catalysed by a Zn complex synthesised from an ethylenediamine Schiff base. The reactions were studied in the 50–130 ◦C range depending on the alcohol, at autogenous pressure. Arrhenius temperature-dependent parameters (activation energies and pre-exponential factors) were estimated for the formation of the lactates. The activation energies (Ea1, Ea2 and Ea 2) for alcoholysis in ethanol were 62.58, 55.61 and 54.11 kJ/mol, respectively. − Alcoholysis proceeded fastest in ethanol in comparison to propanol and butanol and reasonable rates can be achieved in temperatures as low as 50 ◦C. -

Life-Cycle Analysis of Bioproducts and Their Conventional Counterparts in GREET™
ANL/ESD-14/9 Rev. Life-cycle Analysis of Bioproducts and Their Conventional Counterparts in GREET™ Energy Systems Division About Argonne National Laboratory Argonne is a U.S. Department of Energy laboratory managed by UChicago Argonne, LLC under contract DE-AC02-06CH11357. The Laboratory’s main facility is outside Chicago, at 9700 South Cass Avenue, Argonne, Illinois 60439. For information about Argonne and its pioneering science and technology programs, see www.anl.gov. DOCUMENT AVAILABILITY Online Access: U.S. Department of Energy (DOE) reports produced after 1991 and a growing number of pre-1991 documents are available free via DOE’s SciTech Connect (http://www.osti.gov/scitech/) Reports not in digital format may be purchased by the public from the National Technical Information Service (NTIS): U.S. Department of Commerce National Technical Information Service 5301 Shawnee Rd Alexandra, VA 22312 www.ntis.gov Phone: (800) 553-NTIS (6847) or (703) 605-6000 Fax: (703) 605-6900 Email: [email protected] Reports not in digital format are available to DOE and DOE contractors from the Office of Scientific and Technical Information (OSTI): U.S. Department of Energy Office of Scientific and Technical Information P.O. Box 62 Oak Ridge, TN 37831-0062 www.osti.gov Phone: (865) 576-8401 Fax: (865) 576-5728 Email: [email protected] Disclaimer This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor UChicago Argonne, LLC, nor any of their employees or officers, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights.