Report | Nano & Biocidal Silver
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nano-Silver Products Inventory
Petition Appendix A: Nano-Silver Products Inventory Compiled by Center for Food Safety Country of PRODUCT TYPE OF PRODUCT COMPANY WEBSITE Marketing Claim Origin http://www.sourcenaturals.com/products/GP1 Wellness Colloidal Silver™ is produced using a unique electrical process Wellness Colloidal Silver™ Nasal 490 which creates homogeneity, minute particle size, and stability of the Spray Personal Care Source Naturals USA silver particles. http://ciko8.en.ec21.com/Vegetable_Fruits_Cle Removing pesticide residues completely from the fruit and vegetables. aner--1059718_1059768.html Powered by four electric oscillators. Nano-silver/Ozone-Extermination Germs. Washing and sterilizing dishes. No more bacteria such as colon bacillus, salmonella and O-157. Defrosting frozen meat or fish in 5 Vegetable & Fruits Cleaner Cooking 3EVER Co. Ltd Korea minutes. http://www.e- NINK®-Ag series, conductive silver ink series of ABC NANOTECH, are djtrade.com/co/abcnano/GC01567926/CA0156 devised for convenient use of piezoelectricinkjet printing on the various 8109/NINK_Ag_(Silver_Conductive_Ink substrates. NINK®-Ag series aremade up of surface modified nano-silver which is developedby a unique technology of ABC NANOTECH. Fine-pitch conductive lines can be demonstrated on the various plates, such as olycarbonate, polyester, polyimide, and ceramics, by using NINK®-Ag series. Nano-sized silver particles can be sintered at lower temperatures, around 150°C, than microsized silver particles can. NINK®-Ag series are applied to conductive line formation in the shorter process than existing conductive line formation methods." .html) NINK®-Ag Silver Conductive Ink Computer Hardware ABC NanoTech Co, Ltd. Korea http://www.evelinecharles.com/product_detail Nano Silver Cleanser is not a soap, it's a revolution. -

A Silver Nanotechnology Case Study
RISK ANALYSIS OF NANOTECHNOLOGY THROUGH EXPERT ELICITATION: A SILVER NANOTECHNOLOGY CASE STUDY _____________________________________ A Thesis Presented to the faculty of Engineering and Applied Sciences University of Virginia ____________________________________________________ In Partial Fulfillment of the requirements for the Degree Masters of Science Electrical Engineering by Emma Kathryn Fauss August 2008 ii APPROVAL SHEET The thesis is submitted in partial fulfillment of the requirements for the degree of Master of Science Electrical Engineering Emma Kathryn Fauss AUTHOR This thesis has been read and approved by the examining Committee: _________________________ Thesis Advisor _________________________ _________________________ Accepted for the School of Engineering and Applied Science: _________________________ Dean, School of Engineering and Applied Science August 2008 iii Abstract A practical approach is needed to analyze and assess quickly emerging technologies, such as nanotechnologies, where there exist uncertainty and undefined risk. The method presented here utilizes expert elicitation of a pre-selected panel of experts to determine Risk Triggers, which are essential in evaluating a technology, as well as to define exposure scenarios, knowledge gaps and regulatory issues. To illustrate this method, a case study was undertaken on silver nanotechnology in which the research team created a Silver Nanotechnology Consumer Inventory (SNCI), the interactional expert elicited a sample of eleven experts and the research team performed an analysis on the above-mentioned criteria. The interactional expert is key to oversee the elicitation process and the use of Trading Zones among various specializations. Overall this method proved to be very successful in assessing and analyzing the risk of an emerging technology because of the breadth of the expertise elicited, the expedited analysis and the ability to direct research funds where they will be most effective. -
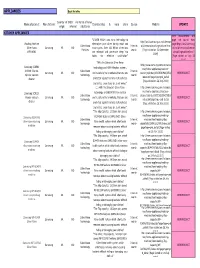
APPLIANCES Back to Intro
APPLIANCES Back to intro Country of Order via Name of nano- Name of product Manufacturer Charateristics & nano claim Source Website UPDATE origin internet substance KITCHEN APPLIANCES Item discontinued and “SILVER WASH uses nano technology to page not found. New http://ww2.samsung.co.za/silvernan Washing Machine electrolyze pure silver during wash and page:http://ww2.samsung Silver Nano Internet o/silvernano/washingmachine.html Silver Nano Samsung KR NO rinse cycles. Over 400 billion silver ions .co.za/silvernano/silvernan Particles search [Page visited on 30 September WFJ145NS are released and penetrate deep into o/washingmachine.html 2009] fabric for effective sanitization” [Page visited on July 23 2010] "With the Silencio’s Silver Nano http://www.samsung.com/uk/consu Samsung SC9540 technology and HEPA filtration system, mer/home-appliances/vacuum- 1800W Silencio Silver Nano Internet Samsung KR NO you’re safe in the knowledge that you are cleaner/cylinders/VCC9540H4K/XEU/ NEW PRODUCT cylinder vacuum technology search index.idx?pagetype=prd_detail cleaner protected against harmful dust particles [Page visited on 28 July 2010] and mites, even if you are a pet owner" (…) with the Silencio’s Silver Nano http://www.samsung.com/ie/consu mer/home-appliances/vacuum- Samsung SC9120 technology and HEPA filtration system Silver Nano Internet cleaner/cylinders/VCC9120V4C/XEU/ silencio vacuum Samsung KR NO you’re safe in the knowledge that you are NEW PRODUCT technology search index.idx?pagetype=prd_detail cleaner protected against harmful dust particles -

CNS-UCSB Traveling Technologies Research Template
CNS-UCSB Traveling Technologies Template © 2009 CNS-UCSB Traveling Technologies Research Template Example Value Chain Analysis for Nanosilver Dr. Stacey Frederick This is the Research Template Steps & Resources, completed for nanosilver. Note that references are used to keep track of where the information comes from. You will use many different data sources, so be sure to keep track of what data is from which source. Things to identify before you begin: Keywords and various spelling of your product: Nanosilver, Nano silver, nano-silver, Silver nanoparticles, Ag Frames of reference: o Type of chain: tangible supply chain o Supply chain position: raw material: nanomaterial forward o Phase: Bulk vs. nanomaterial . Nanoscale metals are often too costly to replace their conventional counterparts except in less price-sensitive applications such as rocket fuel, high-end health care applications, or where load levels are low and cost is less critical (Freedonia 2009). Metal nanoparticles have yet to show that they’re a significant improvement over current materials in many applications, or to become available at prices, which make them economically viable alternatives (Lux 2006). Nanosilver over bulk material: by being nano in scale, it is smaller than the bacteria and thus can block them instead of just disabling them. Innovation to commercialization process phase: Phase III: mass production (Lux 2004) Classification systems to identify products and firms: o North American Industrial Classification System (NAICS): 212222: Silver Ore Mining o International Patent Classification (IPC) System: version. 2009.01: . Section: C: Subsections: Chemistry; Metallurgy (Ag (silver) is a noble metal) . Class: C22: Metallurgy; ferrous or non-ferrous alloys; treatment of alloys or non- ferrous metals . -

Nanotechnology Coatings Coatings Containing Nano-Particles Are
Nanotechnology Coatings Coatings containing nano-particles are already being used in some electrical products. Coatings developed for anti-microbial properties generally contain silver nano-particles. Silver has natural anti-bacterial and anti-fungal properties and silver engineered into nano-particle size increases the surface area in contact with micro-organisms which, in turn, improves its bacterial and fungicidal effectiveness (Nanotech Plc 2006). Products using silver nano-particle coatings include: • Daewoo refrigerator – using “Nano Silver Poly technology”, in which particles of silver are mixed in plastic resin. It is applied to major parts of the refrigerator in order to restrain the growth and increase of a wide variety of bacteria and to suppress odours (Daewoo 2006). • Daewoo vacuum cleaner – the vacuum cleaner has a nanosilver-coated „cyclone canister‟ that allegedly has the effect of removing bacteria and a plethora of dust particles, inhibiting odour, allergy-inducing spores, and other harmful debris (Daewoo 2006). • Daewoo washing machine – again uses Nano Poly Technology by which, according to the manufacturer, “many hurtful bacteria in clothes shall be sterilized perfectly” (Daewoo 2006). • Antibacterial mobile phones – LG Electronics use a Nano Silver antibacterial coating on their mobile phone (Woodrow Wilson International Centre for Scholars 2006). The Motorola i870 mobile phone has an anti-bacterial coating made from silver zeolite nanoparticles (Motorola 2005). • Germ free wireless laser mouse by IOGEAR Inc. - coated with a titanium dioxide and silver nanoparticle compound (Woodrow Wilson International Centre for Scholars 2006). According to the manufacturer, “the special coating protects users from bacteria and germs by neutralizing the harmful microbes on the mouse”. -
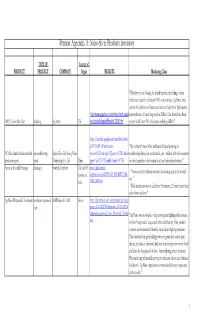
Petition Appendix A: Nano-Silver Products Inventory
Petition Appendix A: Nano-Silver Products Inventory TYPE OF Country of PRODUCT PRODUCT COMPANY Origin WEBSITE Marketing Claim "Whether you are looking for health benefits, the feeling of extra freshness or just the feeling of 100% cotton sheets, AgActive sheet sets are the definition of luxury and can also help in the fight against http://www.agactive.co.uk/index.cfm/fuseact cross infection of super bugs such as MRSA. Our sheets have been 100% Cotton Sheet Set bedding AgActive UK ion/product.display/Product_ID/8/.htm proven to kill over 99% of bacteria including MRSA." http://translate.google.com/translate?u=htt p%3A%2F%2Fwww.nano- "My company's nano-silver antibacterial liquid spraying air- AC filter liquid antibacterial and air conditioning Quan Zhou Hu zheng Nano cn.com%2Fview.asp%3Fprono%3D112&lan conditioning filters, has antibacterial, anti - mildew, with the exception deodorant spray spray Technology Co. Ltd China gpair=zh-CN%7Cen&hl=en&ie=UTF8 of odor, harmless to the human body and other characteristics." Acticoat Wound Dressings bandages Smith & Nephew UK, but US http://global.smith- - "Acticoat destroys bacteria within the dressing and at the wound division as nephew.com/us/ACTICOAT_PRODUCT_RA site." well NGE_8803.htm - "Kills bacteria in vitro in as little as 30 minutes, 2-5 times faster than other forms of silver." Ag Nano Phytoncide Toothpaste toothpaste/personal SH Pharma Co. Ltd Korea http://shpharma.en.ec21.com/product_detail.jsp? care group_id=GC02232811&product_id=CA022328 86&product_nm=Ag_Nano_Phytoncide_Toothp "Ag Nano was developed to keep strong germ fighting effectiveness aste of silver longer and it keeps teeth white and healthy.