Download (2MB)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Monitoring Program for Biodiversity and Non-Indigenous Species in Egypt
UNITED NATIONS ENVIRONMENT PROGRAM MEDITERRANEAN ACTION PLAN REGIONAL ACTIVITY CENTRE FOR SPECIALLY PROTECTED AREAS National monitoring program for biodiversity and non-indigenous species in Egypt PROF. MOUSTAFA M. FOUDA April 2017 1 Study required and financed by: Regional Activity Centre for Specially Protected Areas Boulevard du Leader Yasser Arafat BP 337 1080 Tunis Cedex – Tunisie Responsible of the study: Mehdi Aissi, EcApMEDII Programme officer In charge of the study: Prof. Moustafa M. Fouda Mr. Mohamed Said Abdelwarith Mr. Mahmoud Fawzy Kamel Ministry of Environment, Egyptian Environmental Affairs Agency (EEAA) With the participation of: Name, qualification and original institution of all the participants in the study (field mission or participation of national institutions) 2 TABLE OF CONTENTS page Acknowledgements 4 Preamble 5 Chapter 1: Introduction 9 Chapter 2: Institutional and regulatory aspects 40 Chapter 3: Scientific Aspects 49 Chapter 4: Development of monitoring program 59 Chapter 5: Existing Monitoring Program in Egypt 91 1. Monitoring program for habitat mapping 103 2. Marine MAMMALS monitoring program 109 3. Marine Turtles Monitoring Program 115 4. Monitoring Program for Seabirds 118 5. Non-Indigenous Species Monitoring Program 123 Chapter 6: Implementation / Operational Plan 131 Selected References 133 Annexes 143 3 AKNOWLEGEMENTS We would like to thank RAC/ SPA and EU for providing financial and technical assistances to prepare this monitoring programme. The preparation of this programme was the result of several contacts and interviews with many stakeholders from Government, research institutions, NGOs and fishermen. The author would like to express thanks to all for their support. In addition; we would like to acknowledge all participants who attended the workshop and represented the following institutions: 1. -
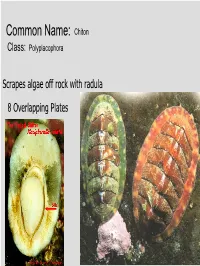
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Environment for Development Improving Utilization of the Queen
Environment for Development Discussion Paper Series December 2020 ◼ EfD DP 20-39 Improving Utilization of the Queen Conch (Aliger Gigas) Resource in the Colombian Caribbean A Bioeconomic Model of Rotational Harvesting Jorge Marco, Diego Valderrama, Mario Rueda, and Maykol R o dr i g ue z - P r i et o Discussion papers are research materials circulated by their authors for purposes of information and discussion. They have not necessarily undergone formal peer review. Central America Chile China Research Program in Economics and Research Nucleus on Environmental and Environmental Economics Program in China Environment for Development in Central Natural Resource Economics (NENRE) (EEPC) America Tropical Agricultural Research and Universidad de Concepción Peking University Higher Education Center (CATIE) Colombia Ghana The Research Group on Environmental, Ethiopia The Environment and Natural Resource Natural Resource and Applied Economics Environment and Climate Research Center Research Unit, Institute of Statistical, Social Studies (REES-CEDE), Universidad de los (ECRC), Policy Studies Institute, Addis and Economic Research, University of Andes, Colombia Ababa, Ethiopia Ghana, Accra India Kenya Nigeria Centre for Research on the Economics of School of Economics Resource and Environmental Policy Climate, Food, Energy, and Environment, University of Nairobi Research Centre, University of Nigeria, (CECFEE), at Indian Statistical Institute, Nsukka New Delhi, India South Africa Tanzania Sweden Environmental Economics Policy Research Environment -

Marine Invertebrate Field Guide
Marine Invertebrate Field Guide Contents ANEMONES ....................................................................................................................................................................................... 2 AGGREGATING ANEMONE (ANTHOPLEURA ELEGANTISSIMA) ............................................................................................................................... 2 BROODING ANEMONE (EPIACTIS PROLIFERA) ................................................................................................................................................... 2 CHRISTMAS ANEMONE (URTICINA CRASSICORNIS) ............................................................................................................................................ 3 PLUMOSE ANEMONE (METRIDIUM SENILE) ..................................................................................................................................................... 3 BARNACLES ....................................................................................................................................................................................... 4 ACORN BARNACLE (BALANUS GLANDULA) ....................................................................................................................................................... 4 HAYSTACK BARNACLE (SEMIBALANUS CARIOSUS) .............................................................................................................................................. 4 CHITONS ........................................................................................................................................................................................... -

Impact of the the COVID-19 Pandemic on a Queen Conch (Aliger Gigas) Fishery in the Bahamas
Impact of the the COVID-19 pandemic on a queen conch (Aliger gigas) fishery in The Bahamas Nicholas D. Higgs Cape Eleuthera Institute, Rock Sound, Eleuthera, Bahamas ABSTRACT The onset of the coronavirus (COVID-19) pandemic in early 2020 led to a dramatic rise in unemployment and fears about food-security throughout the Caribbean region. Subsistence fisheries were one of the few activities permitted during emergency lockdown in The Bahamas, leading many to turn to the sea for food. Detailed monitoring of a small-scale subsistence fishery for queen conch was undertaken during the implementation of coronavirus emergency control measures over a period of twelve weeks. Weekly landings data showed a surge in fishing during the first three weeks where landings were 3.4 times higher than subsequent weeks. Overall 90% of the catch was below the minimum legal-size threshold and individual yield declined by 22% during the lockdown period. This study highlights the role of small-scale fisheries as a `natural insurance' against socio-economic shocks and a source of resilience for small island communities at times of crisis. It also underscores the risks to food security and long- term sustainability of fishery stocks posed by overexploitation of natural resources. Subjects Aquaculture, Fisheries and Fish Science, Conservation Biology, Marine Biology, Coupled Natural and Human Systems, Natural Resource Management Keywords Fisheries, Coronavirus, COVID-19, IUU, SIDS, SDG14, Food security, Caribbean, Resiliance, Small-scale fisheries Submitted 2 December 2020 INTRODUCTION Accepted 16 July 2021 Published 3 August 2021 Subsistence fishing has played an integral role in sustaining island communities for Corresponding author thousands of years, especially small islands with limited terrestrial resources (Keegan et Nicholas D. -

Download Book (PDF)
M o Manual on IDENTIFICATION OF SCHEDULE MOLLUSCS From India RAMAKRISHN~~ AND A. DEY Zoological Survey of India, M-Block, New Alipore, Kolkota 700 053 Edited by the Director, Zoological Survey of India, Kolkata ZOOLOGICAL SURVEY OF INDIA KOLKATA CITATION Ramakrishna and Dey, A. 2003. Manual on the Identification of Schedule Molluscs from India: 1-40. (Published : Director, Zool. Surv. India, Kolkata) Published: February, 2003 ISBN: 81-85874-97-2 © Government of India, 2003 ALL RIGHTS RESERVED • No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any from or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publisher. • -This book is sold subject to the condition that it shall not, by way of trade, be lent, resold hired out or otherwise disposed of without the publisher's consent, in any form of binding or cover other than that in which it is published. • The correct price of this publication is the price printed on this page. Any revised price indicated by a rubber stamp or by a sticker or by any other means is incorrect and should be unacceptable. PRICE India : Rs. 250.00 Foreign : $ (U.S.) 15, £ 10 Published at the Publication Division by the Director, Zoological Survey of India, 234/4, AJ.C. Bose Road, 2nd MSO Building (13th Floor), Nizam Palace, Kolkata -700020 and printed at Shiva Offset, Dehra Dun. Manual on IDENTIFICATION OF SCHEDULE MOLLUSCS From India 2003 1-40 CONTENTS INTRODUcrION .............................................................................................................................. 1 DEFINITION ............................................................................................................................ 2 DIVERSITY ................................................................................................................................ 2 HA.B I,.-s .. .. .. 3 VAWE ............................................................................................................................................ -

SCAMIT Newsletter Vol. 17 No. 10 1999 February
February, 1999 SCAMIT Newsletter Vol. 17, No. 10 SUBJECT: Non-polychaete Taxa from Bight’98 samples: new, odd, problematic GUEST SPEAKER: None - Don Cadien Discussion Leader DATE: 22 March 1999 TIME: 9:30 a.m. to 3:30 p. m. LOCATION: City of San Diego Marine Biololgy Lab 4918 North Harbor Drive #101 San Diego, CA The March meeting will be a first stab at dealing with all the odd and unusual things we non-polychaete workers have encountered so far in analysis of the Bight’98 infaunal samples. Since several different habitats (narrow Channel Islands shelves, bays) were examined, odd things were expected. This first meeting is to find out what areas might need to be addressed by single meetings in future, and to provide an overview of the problems encountered. Bring specimens for help and for others to view and comment on. HELP!! The Bernice P. Bishop Museum in Hawaii is in crisis. Their funding has been cut below the support level for staff and on-going programs, as well as below that necessary for collections Astropecten ornatissimus CSDMWWD, maintenance. The following message station uncertain, circa July 1997 (originating on the TAXACOM list) merits our attention and action. “In response to the request I’ve [Scott Miller] had from various people FUNDS FOR THIS PUBLICATION PROVIDED, IN PART BY THE ARCO FOUNDATION, CHEVRON, USA, AND TEXACO INC. SCAMIT Newsletter in not deemed to be valid publication for formal taxonomic purposes. February, 1999 SCAMIT Newsletter Vol. 17, No.10 about how best to support the problems facing Institution has seen positions eliminated rather Bishop Museum, here is a concise summary than filled [and a change in mission from from Allen Allison. -

Die Arten Der Gattung Aporrhais Da Costa Im Ostatlantik Und
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Drosera Jahr/Year: 1977 Band/Volume: 1977 Autor(en)/Author(s): Cosel von Rudo Artikel/Article: Die Arten der Gattung Aporrhais da Costa im Ostatlantik und Beobachtungen zum Umdrehreflex der Peiikansfuß-Schnecke Aporrhais pespelecani (Mollusca: Prosobranchia) 37-46 DROSERA 77 (2): 37-46 Oldenburg 1977- I X Die Arten der Gattung Aporrhais da Costa im Ostatlantik und Beobachtungen zum Umdrehreflex der Peiikansfuß-Schnecke Aporrhais pespelecani (Mollusca: Prosobranchia) Rudo von Cosel *) Abstract: A short survey with identification key of the four species of the gastropod genus Aporrhais in the East Atlantic is given. — A second type of „turning-back-reflex“ of A. pespelecani (after having put the snail with aper ture upwards), in which the animal bends its extended body round the outer lip and not round the columella as usual, is described and figured for the first time, as well as the observation that the species is able to turn round its shell not only on sandy bottom, but also on hard substrate. Der Pelikansfuß Aporrhais pespelecani (LINNÉ 1758) ist eine der auffallendsten Schneckenarten an der deutschen Nordseeküste und gleichzeitig die Typusart der auf den Atlantik beschränkten Gattung Aporrhais in der Familie Aporrhaidae. Gattung Aporrhais DA COSTA 1778 1778 Aporrhais DA COSTA, Brit. Conch.: 136 1836 Chenopus PHILIPPI, Enum. Moll. Sic., t. I: 215 Gehäuse mit hochgetürmtem Gewinde und Schulterknoten oder Axialrippen. Äußerer Mündungsrand erweitert und zu fingerförmigen Fortsätzen ausgezogen. 5 rezente Arten, davon 2 boreal-lusitanisch-mediterrane und 2 westafrikanische Arten aus der Untergattung Aporrhais, sowie 1 arktisch-carolinische Art {A. -

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Size at Maturation, Spawning Variability and Fecundity in the Queen Conch, Aliger Gigas
Gulf and Caribbean Research Volume 31 Issue 1 2020 Size at Maturation, Spawning Variability and Fecundity in the Queen Conch, Aliger gigas Richard S. Appeldoorn GCFIMembers, [email protected] Follow this and additional works at: https://aquila.usm.edu/gcr Part of the Marine Biology Commons, and the Population Biology Commons To access the supplemental data associated with this article, CLICK HERE. Recommended Citation Appeldoorn, R. S. 2020. Size at Maturation, Spawning Variability and Fecundity in the Queen Conch, Aliger gigas. Gulf and Caribbean Research 31 (1): GCFI 10-GCFI 19. Retrieved from https://aquila.usm.edu/gcr/vol31/iss1/11 DOI: https://doi.org/10.18785/gcr.3101.11 This Gulf and Caribbean Fisheries Institute Partnership is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. VOLUME 25 VOLUME GULF AND CARIBBEAN Volume 25 RESEARCH March 2013 TABLE OF CONTENTS GULF AND CARIBBEAN SAND BOTTOM MICROALGAL PRODUCTION AND BENTHIC NUTRIENT FLUXES ON THE NORTHEASTERN GULF OF MEXICO NEARSHORE SHELF RESEARCH Jeffrey G. Allison, M. E. Wagner, M. McAllister, A. K. J. Ren, and R. A. Snyder....................................................................................1—8 WHAT IS KNOWN ABOUT SPECIES RICHNESS AND DISTRIBUTION ON THE OUTER—SHELF SOUTH TEXAS BANKS? Harriet L. Nash, Sharon J. Furiness, and John W. Tunnell, Jr. ......................................................................................................... 9—18 Volume 31 ASSESSMENT OF SEAGRASS FLORAL COMMUNITY STRUCTURE FROM TWO CARIBBEAN MARINE PROTECTED 2020 AREAS ISSN: 2572-1410 Paul A. -

Malacofauna Marina Del Parque Nacional “Los Caimanes”, Villa Clara, Cuba
Tesis de Diploma Malacofauna Marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Junio, 2011 Universidad Central “Marta Abreu” de Las Villas Facultad Ciencias Agropecuaria TESIS DE DIPLOMA Malacofauna marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Tutor: M. C. Ángel Quirós Espinosa Investigador Auxiliar y Profesor Auxiliar [email protected] Centro de Estudios y Servicios Ambientales, CITMA-Villa Clara Carretera Central 716, Santa Clara Consultante: Dr.C. José Espinosa Sáez Investigador Titular Instituto de Oceanología Junio, 2011 Pensamiento “La diferencia entre una mala observación y una buena, es que la primera es errónea y la segunda es incompleta.” Van Hise Dedicatoria Dedicatoria: A mis padres, a Yandy y a mi familia: por las innumerables razones que me dan para vivir, y por ser fuente de inspiración para mis metas. Agradecimientos Agradecimientos: Muchos son los que de alguna forma contribuyeron a la realización de este trabajo, todos saben cuánto les agradezco: Primero quiero agradecer a mis padres, que aunque no estén presentes sé que de una forma u otra siempre estuvieron allí para darme todo su amor y apoyo. A mi familia en general: a mi abuela, hermano, a mis tíos por toda su ayuda y comprensión. A Yandy y a su familia que han estado allí frente a mis dificultades. Agradecer a mi tutor el M.Sc. Ángel Quirós, a mi consultante el Dr.C. José Espinosa y a la Dra.C. María Elena, por su dedicación para el logro de esta tesis. A mis compañeros de grupo por estos cinco años que hemos compartidos juntos, que para mí fueron inolvidables.