Two Mosaic Gynandromorphs of Automeris Io
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Biology and Distribution of California Hemileucinae (Saturniidae)
Journal of the Lepidopterists' Society 38(4), 1984,281-309 THE BIOLOGY AND DISTRIBUTION OF CALIFORNIA HEMILEUCINAE (SATURNIIDAE) PAUL M. TUSKES 7900 Cambridge 141G, Houston, Texas 77054 ABSTRACT. The distribution, biology, and larval host plants for the 14 species and subspecies of California Hemileucinae are discussed in detail. In addition, the immature stages of Hemileuca neumogeni and Coloradia velda are described for the first time. The relationships among the Hemileuca are examined with respect to six species groups, based on adult and larval characters, host plant relationships and pheromone interactions. The tricolor, eglanterina, and nevadensis groups are more distinctive than the electra, burnsi, or diana groups, but all are closely related. Species groups are used to exemplify evolutionary trends within this large but cohesive genus. The saturniid fauna of the western United States is dominated by moths of the tribe Hemileucinae. Three genera in this tribe commonly occur north of Mexico: Hemileuca, Coloradia, and Automeris. Al though no Automeris are native to California about 50% of the Hemi leuca and Coloradia species in the United States occur in the state. The absence of Automeris and other species from California is due to the state's effective isolation from southern Arizona and mainland Mex ico by harsh mountains, deserts, the Gulf of California, and climatic differences. The Hemileuca of northern Arizona, Nevada, and Utah are very similar to that of California, while those of Oregon, Washing ton, and Idaho represent subsets of the northern California fauna. The majority of the saturniid species in the United States have had little or no impact on man, but some Hemileucinae have been of eco nomic importance. -
![PRODROMUS LEPIDOPTERORUM SLOVACIAE [Prodromus of the Lepidoptera of Slovakia]](https://docslib.b-cdn.net/cover/7872/prodromus-lepidopterorum-slovaciae-prodromus-of-the-lepidoptera-of-slovakia-677872.webp)
PRODROMUS LEPIDOPTERORUM SLOVACIAE [Prodromus of the Lepidoptera of Slovakia]
1965 Journal of the Lepidopterists' Society 81 The time spent in each instar is variable; the following represents an average life cycle. EGG Eggs laid in late June emerge eight to nine days later. FIRST INSTAR Six to seven days. SECOND INSTAR Seven to eight days. THIRD INSTAR Eleven to thirteen days. FOURTH INSTAR About eight and a half months, the hibernation stage covering the period from late August to late the follow ing April. FIFTH INSTAR Ten to thirteen days. FINAL INSTAR Ten days to a little over two weeks (females seem to develop more slowly). PUPA Thirteen to sixteen days. The first adults normally emerging about the start of the second week in June. My thanks go to Dr. John R. Reeder of Yale University for the host plant determination and to William Howe of Ottawa, Kansas, for the illustration of the life history. BOOK REVIEW PRODROMUS LEPIDOPTERORUM SLOVACIAE [Prodromus of the Lepidoptera of Slovakia]. By Karel Hruby. 1964. 962 pp., 3 maps. Pub lished by the Slovak Academy of Sciences. Klemensova Street 27, Bra tislava, Czechoslovakia. Price 83,- Kcs. Slovakia is an interesting and beautiful country in Central Europe. There are a number of different land formations; in the southern part it is the great Lowland of the river of Danube with xerothermic localities, in the north there are the mountains of which the Tatra is the highest (with the Peak of Gerlach 2,663 m) . The fauna of Lepidoptera of Slovakia was intensively investigated, but results of this work were published in different languages and dispersed in short faunistic contributions. -

Io Moth Automeris Io (Fabricius) (Insecta: Lepidoptera: Saturniidae)1 Donald W
EENY608 Io Moth Automeris io (Fabricius) (Insecta: Lepidoptera: Saturniidae)1 Donald W. Hall2 Introduction The beautiful Io moth, Automeris io (Fabricius), is one of our most recognizable moths. It is distinctive because of its prominent hind wing eyespots. The Io moth, like many of the other saturniid moths, is less common now in parts of its range. With the exception of Cape Cod and some of the Massachusetts islands, it is now rare in New England where it was once common, and its populations have declined in the Gulf States (with the exception of Louisiana) since the 1970s (Manley 1993). The attractive Io moth caterpillar is also well-known because of its painful sting. Figure 1. Male Io moth, Automeris io (Fabricius). Automeris is a large genus with about 145 species (Heppner Credits: Donald W. Hall, University of Florida 1996). All Automeris species are characterized by large eyespots in the middle of the hind wings. Most species Synonymy are found in Central and South America. There are seven Fabricius (1775, p.560) described the Io moth and named species in the United States. Five of these, Automeris it Bombyx io. Abbott and Smith (1797, p.97) published zephyria Grote (New Mexico and western Texas), Automeris the first account of the Io moth’s life cycle under the cecrops (Boisduval), Automeris iris (Walker), Automeris name Phalaena io. Some early references used the genus patagoniensis Lemaire, and Automeris randa Druce name Hyperchiria (e.g., Eliot & Soule 1902, Lintner 1872, (southeastern Arizona) are found only in the western U.S. Stratton-Porter 1921, Strecker 1872). -

Extreme Diversity of Tropical Parasitoid Wasps Exposed by Iterative Integration of Natural History, DNA Barcoding, Morphology, and Collections
Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections M. Alex Smith*†, Josephine J. Rodriguez‡, James B. Whitfield‡, Andrew R. Deans§, Daniel H. Janzen†¶, Winnie Hallwachs¶, and Paul D. N. Hebert* *The Biodiversity Institute of Ontario, University of Guelph, Guelph Ontario, N1G 2W1 Canada; ‡Department of Entomology, 320 Morrill Hall, University of Illinois, 505 S. Goodwin Avenue, Urbana, IL 61801; §Department of Entomology, North Carolina State University, Campus Box 7613, 2301 Gardner Hall, Raleigh, NC 27695-7613; and ¶Department of Biology, University of Pennsylvania, Philadelphia, PA 19104-6018 Contributed by Daniel H. Janzen, May 31, 2008 (sent for review April 18, 2008) We DNA barcoded 2,597 parasitoid wasps belonging to 6 microgas- A detailed recognition of species in parasitoid communities is trine braconid genera reared from parapatric tropical dry forest, cloud necessary because of the pivotal role parasitoids play in food web forest, and rain forest in Area de Conservacio´ n Guanacaste (ACG) in structure and dynamics. While generalizations about the effects of northwestern Costa Rica and combined these data with records of parasitoids on community diversity are complex (7), a common- caterpillar hosts and morphological analyses. We asked whether place predictor of the impact of a parasitoid species on local host barcoding and morphology discover the same provisional species and dynamics is whether the parasitoid is a generalist or specialist. A whether the biological entities revealed by our analysis are congruent generalist, especially a mobile one, is viewed as stabilizing food webs with wasp host specificity. Morphological analysis revealed 171 (see ref. -

Pharmacologic Manipulation of the Io Moth Wing Pattern
F1000Research 2017, 6:1319 Last updated: 17 MAY 2019 RESEARCH NOTE Giving eyespots a shiner: Pharmacologic manipulation of the Io moth wing pattern [version 1; peer review: 2 approved with reservations] Andrei Sourakov McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL, 32611, USA First published: 03 Aug 2017, 6:1319 ( Open Peer Review v1 https://doi.org/10.12688/f1000research.12258.1) Latest published: 26 Sep 2017, 6:1319 ( https://doi.org/10.12688/f1000research.12258.2) Reviewer Status Abstract Invited Reviewers Our knowledge of wing pattern formation in Lepidoptera has advanced 1 2 significantly in recent years due to the careful examination of several groups of butterflies. The eyespot is a prominent feature of Lepidoptera wing pattern, especially in the family Saturniidae. The present study version 2 report report examined how sulfated polysaccharides, which are known to simulate cold published shock effect in nymphalid butterflies, affected the wing pattern formation of 26 Sep 2017 the Io moth, Automeris io (Saturniidae). Prepupae and pupae of this species were subjected to injections of heparin and cold shock. While the version 1 cold shock had little to no effect on wing pattern, the aberrations resulting published report report from heparin injections consisted of moderate to profound increases in 03 Aug 2017 melanism around the eyespots. The resulting aberrations are dubbed ‘Black Eye’ and ‘Comet Eye.’ Most other known aberrations of Automeris io Arnaud Martin , The George Washington eyespots are summarized, illustrated and named. 1 University, Washington, USA Keywords Eyespots, wing pattern, Lepidoptera, heparin, phenotypic plasticity, 2 Jeffrey M. -

Io Moth Automeris Io Kingdom: Animalia FEATURES Phylum: Arthropoda the Wingspan of This Species Is Two to Three and Class: Insecta One-Fourth Inches
io moth Automeris io Kingdom: Animalia FEATURES Phylum: Arthropoda The wingspan of this species is two to three and Class: Insecta one-fourth inches. The male and female have Order: Lepidoptera different coloration. For males, the upperside of the wings is yellow with brown spots, and a faint line Family: Saturniidae can be seen in the middle of the forewing. The line ILLINOIS STATUS parallels the edge of the forewing. The upperside of the female’s wings is raspberry colored with common, native markings similar to those of the male. The hindwing of both sexes contains a large black spot with a blue and white center surrounded by a yellow band, then a black line followed by another yellow band. The underside of the male’s wings is yellow, while the underside of the female’s wings is red-brown. The large spot is visible on the hindwing of both sexes. BEHAVIORS The Io moth is found statewide. The adults are active starting in late May and can be found in wooded areas and savannas. The adult does not feed, while the larvae feed on many species of plants. One generation is produced in northern Illinois while two generations are produced annually in the southern part of the state. ILLINOIS RANGE © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. provided by Brett Hondow/pond5.com © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. Aquatic Habitats bottomland forests Woodland Habitats bottomland forests; southern Illinois lowlands; upland deciduous forests Prairie and Edge Habitats edge; shrub prairie © Illinois Department of Natural Resources. -

Lepidoptera, Saturniidae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Nachrichten des Entomologischen Vereins Apollo Jahr/Year: 1997 Band/Volume: 18 Autor(en)/Author(s): Racheli Luigi, Racheli Tommaso Artikel/Article: Further notes on the Saturniidae of Ecuador 173-180 Nachr. entomol. Ver. Apollo, N.F. 18 (2/3): 173-180 (1997) 173 Further notes on the Saturniidae of Ecuador (Lepidoptera, Saturniidae) Luigi Racheli and Tommaso Racheli Luigi Racheli1, Via Fara Sabina 1,1-00199 Rome, Italy Tommaso Racheli, Via G. Valmarana 66,1-00139 Rome, Italy Abstract: Some distributional notes on the Saturniids of Ecuador are report ed, including the first records of Automeris larra (Walker, 1855), Automeris heppneri Lemaire, 1982, and Automerina vala (Kirby, 1871) and for Ecuador. Weitere Anmerkungen zur Saturniidenfauna Ecuadors (Lepidoptera, Saturniidae) Zusammenfassung: Es werden einige Verbreitungsangaben für ecuadoriani- sche Saturniiden besonders aus der Gegend von Napo gegeben, darunter auch die Erstnachweise für Ecuador von Automeris larra (Walker, 1855), Au tomeris heppneri Lemaire, 1982 und Automerina vala (Kirby, 1871). Introduction During a collecting trip in Ecuador made in July/August 1996, various species of Saturniidae were collected especially in the Napo region. In this paper we report additional data to the catalogue of Lemaire & Vene- dictoff (1989) and to the subsequent notes of Racheli (1994, 1995 a, 1995b, 1995c, in press). Automeris larra (Walker, 1855), Automeris heppneri Lemaire, 1982, and Automerina vala (Kirby, 1871) are reported for the first time for Ecuador and additional data are also reported for 29 species including the rare Leucanella apollinairei (Dognin, 1923) and Leucanella maasseni (Moschler, 1872). -

Io Moth (Automeris Io)
CLOSE ENCOUNTERS WITH THE ENVIRONMENT What’s Eating You? Io Moth (Automeris io) Eric W. Hossler, MD; Dirk M. Elston, MD; David L. Wagner, PhD f the 7 species of Automeris moths (order, About 10 days after being deposited, minute 2- to Lepidoptera; family, Saturniidae) found in the 3-mm larvae emerge from the ova and feed gregari- O United States, Automeris io often is the most ously upon their host plant. Favorite foods of the Io common and familiar. Its range extends as far north caterpillars include azaleas, birch, blackberry, cherry, as Quebec, Ontario, and southern Manitoba, Canada; clover, cotton, currant, elm, hackberry, hibiscus, west to Utah, Colorado, Nebraska, and Texas; and mesquite, oak, pear, poplar, redbud, rose, sassafras, south to Florida, eastern Mexico, and Costa Rica.1-3 and willow.1,2,4 In addition, larvae frequently feed on Across much of its range, the moths and their lar- grasses such as corn or Bermuda grass.2,4 Io moths are vae are among the most common giant silk moths around in deciduous woodlands, forests, and fields; encountered by the public. In Louisiana, the closely along power line rights-of-way; and in orchards, related Automeris louisiana largely replaces the Io parks, and suburban yards.2,5 moth in coastal areas.2 The sexually dimorphic adults have a wingspan The Io moth has 4 life stages: egg, larva (or cat- of 2.0 to 3.5 in and are easily recognized by the pres- erpillar), pupa, and adult. Eclosion of Io moths from ence of prominent black to blue eyespots with white cocoons occurs during late morning or early evening. -

Special Edition: Moths Interview with Bart Coppens, Guest Speaker at ICBES 2017
INTERNATIONAL ASSOCI ATION OF BUTTERFLY EXHIBITORS AND SUPPL IERS Volume 16 Number 3 MAI– JUNE 2017 Visit us on the web at www.iabes.org Special edition: moths Interview with Bart Coppens, guest speaker at ICBES 2017 Who are you? I’m Bart Coppens (24) from the Netherlands – a fervent breeder of moths and aspiring entomologist. In my home I breed over 50 species of moths (mainly Saturniidae) on yearly basis. My goal is to expand what started out as a hobby into something more scientific. It turns out the life cycle and biology of many Saturni- idae is poorly known or even unrecorded. By importing eggs and cocoons of rare and obscure species and breeding them in cap- tivity I am able to record undescribed larvae, host plants and the life history of several moth species – information that I publish on a scientific level. My ambition is also to gradually get into more difficult subjects such as the taxonomy, morphology and evolution and perhaps even the organic chemistry (in terms of defensive chemicals) of Saturniidae – but for now these subjects are still beyond my le- Bart with Graellsia isabella vel of comprehension, as relatively young person that has not yet completed a formal education. I’d also like to say I have a general passion for all kinds of Lepidoptera, from butterflies to the tiniest species of moths, I truly like all of them. The reason I mention Saturniidae so much is because I have invested most of my time and expertise into this particular family of Lepidoptera, simply because this order of insects is too big to study on a general scale, so I decided to specialise myself a little in the kinds of moths I find the most impressi- ve and fascinating myself – and was already the most familiar with due to my breeding hobby. -

Hemileucinae (Lepidoptera: Saturniidae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Racheli, L.; Racheli, T. An update checklist of the Saturniidae of Ecuador. Part I: Hemileucinae (Lepidoptera: Saturniidae) SHILAP Revista de Lepidopterología, vol. 33, núm. 130, junio, 2005, pp. 203-223 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45513013 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 203-223 An update 13/6/77 18:37 Página 203 SHILAP Revta. lepid., 33 (130), 2005: 203-223 SRLPEF ISSN:0300-5267 An update checklist of the Saturniidae of Ecuador. Part I: Hemileucinae (Lepidoptera: Saturniidae) L. Racheli & T. Racheli Abstract In this paper, an update checklist of 199 taxa belonging to the subfamily Hemileucinae (Saturniidae) recorded for Ecuador is compiled. It includes all the available distributional data reviewed in the literature and additional records from material examined in private and public collections. All the provincial records are listed for each taxon. For some uncommon or little-known species, further details on their distributions are given. The current taxonomic arrangements of some species-groups are discussed. Furthermore, the female of Meroleuca (Meroleucoides) albomaculata (Dognin, 1916) is described for the first time and Molippa placida (Schaus, 1921) is reported for the first time for Peru. KEY WORDS: Lepidoptera, Saturniidae, Hemileucinae, checklist, Ecuador. Una lista actualizada de los Saturniidae del Ecuador. -

Lepidoptera: Saturniidae, Hemileucinae
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Galathea, Berichte des Kreises Nürnberger Entomologen e.V. Jahr/Year: 2004 Band/Volume: 15_Supp Autor(en)/Author(s): Decaens Thibaud Artikel/Article: Description of a new Automeris from Bolivia (Lepidoptera: Saturniidae, Hemileucinae) 69-76 ©Kreis Nürnberger Entomologen; download unter www.biologiezentrum.at galathea» Berichte des Kreises Nürnberger Entomologen • Supplement 15 • 2004 Description of a new Automeris from Bolivia (Lepidoptera: Saturniidae, Hemileucinae). Thibaud Decaens Abstract: A new Automeris species is described from Bolivia. A. sylviae new species is closely related to A. duchartrei, from which it differs by the omamentation and colour pattern of the wings. It is probably a low elevation Andean forest species. The holotype S will be deposited in the Museum national d’Histoire naturelle of Paris, France. Resume: Un nouvel Automeris est decrit de Bolivie. A. sylviae nov. species est proche de A. duchartrei dont il differe morphologiquement par l’omementation et la couleur des ailes. II s’agit probablement d’un representants de la faune des forets andines de basse altitude. L’holotype <S est depose au Museum national d’Histoire naturelle de Paris, France. Resumen: Se describe un nuevo Automeris boliviano. A. sylviae nov. species es cercano de A. duchartrei del cual se distingue por la omamüentacion y el colour de las alas. Esa especie pertenece probablemente a la fauna silvatica de la cordillera andina de baja altura. El holotipo S de depositara en el Museum national d’Histoire naturelle de Paris, Francia. Zusammenfassung: Eine neue Automeris-Axt aus Bolivien wird beschrieben. -
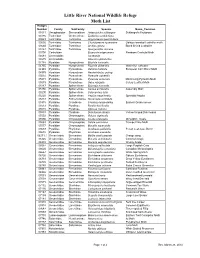
Little River NWR Moth List
Little River National Wildlife Refuge Moth List Hodges Number Family SubFamily Species Name_Common 01011 Oecophoridae Stenomatinae Antaeotricha schlaegeri Schlaeger's Fruitworm 03186 Tortricidae Olethreutinae Epiblema scudderiana 03623 Tortricidae Tortricinae Argyrotaenia quercifoliana 03635 Tortricidae Tortricinae Choristoneura rosaceana Oblique-banded Leafroller moth 03660 Tortricidae Tortricinae Archips grisea Black Shield Leafroller 03727 Tortricidae Tortricinae Sparganothis niveana 03790 Cochylidae Eugnosta erigeronana Fleabane Cochylid Moth 04681 Limacodidae Isa textula 04685 Limacodidae Adoneta spinuloides 04748 Pyralidae Nymphulinae Elophila icciusalis 04755 Pyralidae Nymphulinae Elophila obliteralis Waterlily Leafcutter 04949 Pyralidae Pyraustinae Ostrinia nubilalis European Corn Borer Moth 04979 Pyralidae Pyraustinae Neohelvibotys polingi 05034 Pyralidae Pyraustinae Pyrausta signatalis 05071 Pyralidae Pyraustinae Pyrausta acrionalis Mint-loving Pyrausta Moth 05079 Pyralidae Pyraustinae Udea rubigalis Celery Leaftier Moth 05147 Pyralidae Spilomelinae Epipagis huronalis 05150 Pyralidae Spilomelinae Samea ecclesialis Assembly Moth 05200 Pyralidae Spilomelinae Colomychus talis 05226 Pyralidae Spilomelinae Palpita magniferalis Splendid Palpita 05313 Pyralidae Schoenobiinae Donacaula sordidella 05378 Pyralidae Crambinae Crambus laqueatellus Eastern Grass-veneer 05512 Pyralidae Pyralinae Pyralis disciferalis 05518 Pyralidae Pyralinae Aglossa cuprina 05533 Pyralidae Pyralinae Dolichomia olinalis Yellow-fringed Dolichomia 05552 Pyralidae