Expression of CD4 in Transgenic Mice Alters the Specificity of CD8 Cells For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jessie J.Pdf
Jessie J Jessica Ellen Cornish was born on 27th of March 1988 in Redbridge, London in UK. Her stage name is Jessie J. Jessie began singing with 11 years in showbusiness. She appeared in a West End production of Whistle Down the Wind by Andrew Lloyd Webber. She attended Colin’s Performing Arts School. At the age of 16 she began studying musical theater at the BRIT School, and joined a girl group called Soul Deep at the age of 17. She sang there for 2 years and then she left the group and became an independent singer. Young Jessie J When she was 17 she wrote her first song, Big white room, which has become a signature song for her. She wrote it after she was hospitalized while sharing a room with a younger boy who later sadly lost his life. Her life is very ordinary. She is the youngest of three children and she is a singer and songwriter. She sings R&B, soul, pop and hip – hop. Her father is a social worker and her mother is kindergarten teacher. Her sister Hanna is a fotographer and her second sister Rachel is a childminder. 2006–2009: Career beginnings She had signed on with now-defunct Gut Records, having fallen to bankruptcy. She now has a publishing arrangement with SONY ATV and shows great promise as a writer. Her song, Sexy Silk, was picked by Nivea for a campaign. She has also written for Chris Brown and Lisa Lois. Jessie J was responsible for making Miley Cyrus’ song’s, one of them is Party In The USA which is one of her greatests hits. -

Marriott's Oceana Palms |Singer Island, Riviera Beach, Florida
Marriott’s Oceana Palms | Singer Island, Riviera Beach, Florida Week 2020 Check-In Days 2021 Check-In Days Season No. Thursday Friday Saturday Sunday Thursday Friday Saturday Sunday 1 Jan 2 – Jan 9 Jan 3 – Jan 10 Jan 4 – Jan 11 Jan 5 – Jan 12 Jan 7 – Jan 14 Jan 1 – Jan 8 Jan 2 – Jan 9 Jan 3 – Jan 10 2 Jan 9 – Jan 16 Jan 10 – Jan 17 Jan 11 – Jan 18 Jan 12 – Jan 19 Jan 14 – Jan 21 Jan 8 – Jan 15 Jan 9 – Jan 16 Jan 10 – Jan 17 3 Jan 16 – Jan 23 Jan 17 – Jan 24 Jan 18 – Jan 25 Jan 19 – Jan 26 Jan 21 – Jan 28 Jan 15 – Jan 22 Jan 16 – Jan 23 Jan 17 – Jan 24 PLATINUM 4 Jan 23 – Jan 30 Jan 24 – Jan 31 Jan 25 – Feb 1 Jan 26 – Feb 2 Jan 28 – Feb 4 Jan 22 – Jan 29 Jan 23 – Jan 30 Jan 24 – Jan 31 5 Jan 30 – Feb 6 Jan 31 – Feb 7 Feb 1 – Feb 8 Feb 2 – Feb 9 Feb 4 – Feb 11 Jan 29 – Feb 5 Jan 30 – Feb 6 Jan 31 – Feb 7 6 Feb 6 – Feb 13 Feb 7 – Feb 14 Feb 8 – Feb 15 Feb 9 – Feb 16 Feb 11 – Feb 18 Feb 5 – Feb 12 Feb 6 – Feb 13 Feb 7 – Feb 14 PLATINUM PLUS 7 Feb 13 – Feb 20 Feb 14 – Feb 21 Feb 15 – Feb 22 Feb 16 – Feb 23 Feb 18 – Feb 25 Feb 12 – Feb 19 Feb 13 – Feb 20 Feb 14 – Feb 21 PRESIDENTS DAY 8 Feb 20 – Feb 27 Feb 21 – Feb 28 Feb 22 – Feb 29 Feb 23 – Mar 1 Feb 25 – Mar 4 Feb 19 – Feb 26 Feb 20 – Feb 27 Feb 21 – Feb 28 9 Feb 27 – Mar 5 Feb 28 – Mar 6 Feb 29 – Mar 7 Mar 1 – Mar 8 Mar 4 – Mar 11 Feb 26 – Mar 5 Feb 27 – Mar 6 Feb 28 – Mar 7 10 Mar 5 – Mar 12 Mar 6 – Mar 13 Mar 7 – Mar 14 Mar 8 – Mar 15 Mar 11 – Mar 18 Mar 5 – Mar 12 Mar 6 – Mar 13 Mar 7 – Mar 14 11 Mar 12 – Mar 19 Mar 13 – Mar 20 Mar 14 – Mar 21 Mar 15 – Mar 22 Mar 18 – Mar 25 Mar 12 – -
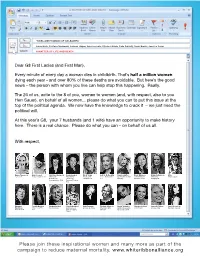
Please Join These Inspirational Women and Many More As Part of the Campaign to Reduce Maternal Mortality
‘WIVES AND HUSBAND OF G8 LEADERS’ Laura Bush, Svetlana Medvedev, Laureen Harper, Veronica Lario, Kiyoko Fukuda, Carla Sarkozy, Sarah Brown, Joachim Sauer A MATTER OF LIFE AND DEATH Dear G8 First Ladies (and First Man), Every minute of every day a woman dies in childbirth. That’s half a million women dying each year - and over 80% of these deaths are avoidable. But here’s the good news – the person with whom you live can help stop this happening. Really. The 26 of us, write to the 8 of you, women to women (and, with respect, also to you Herr Sauer), on behalf of all women... please do what you can to put this issue at the top of the political agenda. We now have the knowledge to crack it – we just need the political will. At this year’s G8, your 7 husbands (and 1 wife) have an opportunity to make history here. There is a real chance. Please do what you can – on behalf of us all. With respect, Emma Thompson Annie Lennox Christiane Amanpour Donna Langley Kirsty Young Lady de Rothschild Claudia Schiffer Wendi Murdoch Elizabeth Edwards Misia Actress, UK Singer, Songwriter, UK CNN Chief President of Broadcaster, CE, LE Rothschild Supermodel, Actress, Chief Strategist, Attourney, Singer, Japan International Production, Presenter, UK LLC Germany Myspace China Campaigner Correspondent, USA Universal Pictures, USA Margaret Fiorella Mannoia Margherita Hack Claudie Haigneré Katja Riemann Elisabeth Murdoch Christy Turlington Christine Ockrent Gwyneth Paltrow Sarah McLachlan MacMillan Singer, Songwriter, Scientist, Italy Astronaut and Actress, Germany Chairman, CEO, CARE Ambassador, COO, France Actress, USA Musician, Canada Historian, USA Italy Advisor to ESA, UK/USA Supermodel, USA France Please join these inspirational women and many more as part of the campaign to reduce maternal mortality. -

Press Release
PRESS RELEASE United Nations Secretary-General appoints the Japanese singer MISIA as Honorary Ambassador for the 2010 United Nations Biodiversity Conference Montreal, 1 March 2010. United Nations Secretary-General Ban Ki-moon has appointed the Japanese female singer and songwriter, MISIA, as the Honorary Ambassador for the forthcoming tenth meeting of the Conference of the Parties to the Convention on Biological Diversity, which will be held from 18 to 29 October 2010 in Nagoya, Aichi Prefecture, Japan. In her honorary role, MISIA will help raise awareness of the continuing depletion of biological resources and inform the public on the sustainable use of biodiversity. “In this International Year of Biodiversity, we must counter the perception that people are disconnected from our natural environment. We must increase understanding of the implications of losing biodiversity,” said Secretary-General Ban Ki-moon. Referring to Honorary Ambassador MISIA, the Secretary-General said she could “help highlight our common struggles for protecting life on Earth and the actions we can take to slow down biodiversity loss.” Throughout her successful career, MISIA has been an advocate of sustainable development. Through her performances, she has worked to raise public awareness on the United Nations Millennium Development Goals and, in 2008, she established a voluntary organization, Child AFRICA, to support education in Africa. This honorary appointment will help bring much needed attention to the importance of biodiversity and the urgent need for progress to meet the three objectives of the Convention on Biological Diversity (CBD). On learning of her appointment, MISIA said, “While travelling to different parts of the world, I have realized that our life and culture vary a lot and this diversity has been nurtured by the rich natural environments of different regions. -

Chinese Music Reality Shows: a Case Study
Chinese Music Reality Shows: A Case Study A Thesis Submitted to the Faculty of Drexel University by Zhengyuan Bi In partial fulfillment of the requirements for the degree of Master of Science in Television Management January 2017 ii © Copyright 2017 Zhengyuan Bi All Right Reserved. iii DEDICATION I dedicate this thesis to my family and my friends, with a special feeling of gratitude to my loving parents, my friends Queena Ai, Eileen Zhou and Lili Mao, and my former boss Kenny Lam. I will always appreciate their love and support! iv ACKNOWLEDGMENTS I would like to take this opportunity to thank my thesis advisor Philip Salas and Television Management Program Director Al Tedesco for their great support and guidance during my studies at Drexel University. I would also like to thank Katherine Houseman for her kind support and assistance. I truly appreciate their generous contribution to me and all the students. I would also like to thank all the faculty of Westphal College of Media Arts & Design, all the classmates that have studied with me for these two years. We are forever friends and the best wishes to each of you! v Table of Contents DEDICATION ………………………………………………………………………………..iii ACKNOWLEDGEMENTS…………………………………………………………………...iv ABSTRACT……………………………………………………………………………………vii Chapter 1: Introduction………………………………………………………………………..1 1.1 Introduction………………………………………………………………………………...3 1.2 Statement of the Problem…………………………………………….................................3 1.3 Background…………………………………………………………………………………4 1.4 Purpose of the study…………………………………………………………………….....5 -

Body As Evidence
Chapter 1 Pop Goes Democracy Mediating Race, Gender, and Nation on American Idol Parallels between presidential elections and the hit TV series are not hard to make. Indeed, they had become punch lines in comedyAmerican routines: Idol from Comedy Central’s Stephen Colbert complaining that it took too long to determine the next American Idol (in a gag that had audiences assuming he was referring to the next U.S. president) to ABC’s Jimmy Kimmel quipping that, after watching the vice presidential debate during the U.S. presidential campaign in 2008, he “voted four times for Sarah Palin and six times for David Archuleta,” in reference to the popular Republican vice presidential candidate and frontrunner contestant that year. Interestingly, movie critic RogerAmerican Ebert Idol’s mounted criticism against Palin for being “the American Idol candidate,” in which he argued the following: I think I might be able to explain some of Sarah Palin’s appeal. She’s the “American Idol” candidate. Consider. What defines an “American Idol” finalist? They’re good‑looking, work well on television, have a sunny personality, are fierce competitors, and so talented, why, they’re darned near the real thing. There’s a reason “American Idol” gets such high ratings. People identify with the contestants. They think, Hey, that could be me up there on that show! . My problem is, I don’t want to be up there. I don’t want a vice president who is darned near good enough. I want a vice president who is better, wiser, well‑traveled, has met world leaders, who three months ago had an opinion on Iraq. -

A Fresh Start for Forests, a Fresh Start for Biodiversity
TOPICS ALL PHOTOS MASATOSHI SAKAMOTO MASATOSHI ALL PHOTOS Representatives from national governments, international organizations and NGOs gather at the bridging ceremony linking the International Year of Biodiversity with the From December 18 to 20, 2010, a closing event International Year of Forests for the International Year of Biodiversity was held, Fmainly in the city of Kanazawa, Ishikawa Prefecture. The Closing Ceremony held on December 18 A Fresh brought together about 400 participants from twenty-nine countries and regions, including Minister of the Environment Ryu Matsumoto, Minister of Start for Agriculture, Forestry and Fisheries Michihiko Kano, Executive Secretary of the Convention on Biological Forests, Diversity Ahmed Djoghlaf, and other officers and representatives of the governments of various coun- tries, international organizations and NGOs. A Fresh In the first and second parts of the ceremony, leading representatives gave speeches on the results Start for achieved at the tenth Conference of the Parties to the Convention on Biological Diversity (COP10) held in Nagoya, Aichi Prefecture in October, 2010. Minister Biodiversity Matsumoto, who presided over COP10, said, “Thanks Highlighting Japan FEBRUARY 2011 Highlighting Japan FEBRUARY 2011 20 21 to the cooperation of so many of you, we were able to achieve numerous re- sults, including the Aichi Target and Nagoya Protocol. In retrospect, the 2010 International Year of Biodiversity turned out to be a very fruitful year for global biodiversity, and an unforgettable one for me.” Afterwards, a bridging ceremony to the 2011 International Year of Forests was held. Jan McAlpine, director of the United Participants in the panel discussion held as part of the Nations Forum on Forests, said, “The Commemorative Symposium ceremony marks the starting point, not only for forests but also for biodiversity.” McAlpine International Year of Forests. -

Grammy Nominated Music Sensation Jessie J Announced As Headline Entertainer for Times Square New Year’S Eve Live, Commercial-Free Webcast
Grammy Nominated Music Sensation Jessie J Announced as Headline Entertainer for Times Square New Year’s Eve Live, Commercial-Free Webcast Release Date: Dec 17, 2015 Jessie J to Perform John Lennon’s “Imagine” and Many of Her Top Hits; Singer-Songwriter Daya to Perform Hit Song “Hide Away” Webcast Available to Embed for Digital Media Outlets, Bloggers, Social Media Editors, Webmasters, and More New York, N.Y. (December 17, 2015)—Times Square Alliance and Countdown Entertainment, co-organizers of New Year’s Eve in Times Square, today announced that Jessie J will perform in Times Square on New Year’s Eve as the headliner of the live commercial-free webcast. The seventh annual webcast, enabled by Livestream, will cover the action and festivities in Times Square, beginning with the Ball Raising at 6 p.m. EST, plus live musical performances, hourly countdowns, behind-the-scenes stories, and star-studded interviews as anticipation builds towards the midnight countdown and the famous Ball Drop. International music sensation and Grammy Award nominee Jessie J will perform several of her top hits throughout the night and will continue the New Year’s Eve tradition of singing John Lennon’s “Imagine” moments before midnight. The British artist is best known for her wide vocal range and unconventional performance style, mixing soul vocals with contemporary, hip- hop and pop. Additionally, singer-songwriter Daya will perform her hit song “Hide Away.” Univision will present musical performances by Maluma and CNCO, winners of Univision’s hit reality show La Banda. Last year’s webcast was watched by more than 1.7 million unique viewers representing nearly 200 countries. -

Global Entrepreneurship Monitor
Global Entrepreneurship Monitor AUTHORS Niels Bosma Thomas Schøtt Siri Terjesen Penny Kew ABOUT THE AUTHORS Niels Bosma Niels Bosma is an assistant professor with the Utrecht University School of Economics, co-ordinator of the Utrecht University Social Entrepreneurship Initiative and GEM member for the Netherlands and Belgium. Niels is particularly interested in individuals’ entrepreneurial and innovative behaviour in different regional and social contexts, and has published in various academic journals and co-authored several GEM global and special reports. He is also a member of the board of advisors with the Global Entrepreneurship Research Association and academic member of GECES, an expert group advising the European Commission on their Social Business Initiative. Thomas Schøtt Thomas Schøtt is Professor of Entrepreneurship at the University of Southern Denmark and adjunct Professor of Entrepreneurship at Tehran University. Thomas has been leading GEM in Denmark and is a member of the GEM teams in Iran, Turkey, Nigeria, and Bosnia and Herzegovina. He has co-authored special GEM reports on education and training, and on youth. Recent GEM- based articles are in the International Journal of Gender and Entrepreneurship, Small Business Economics and Research Policy. Thomas often runs GEM training workshops around the world, with the next workshops planned for Turkey, China, Mexico and Uganda. Siri Terjesen Siri Terjesen is Chair of Entrepreneurship at American University’s Kogod School of Business and professor at the Norwegian School of Economics. Siri has been a member of the GEM Hungary team since 2005 and co-authored the initial GEM Social Entrepreneurship Study in 2009. Her research on comparative international entrepreneurship, including GEM-based work, has been published in the Journal of Management, Entrepreneurship Theory & Practice, Strategic Entrepreneurship Journal, Small Business Economics, Venture Capital, and other leading journals. -

Japanese Singer Misia Appointed Honorary Ambassador for Tenth Conference of Parties to Un Convention on Biodiversity, in Nagoya, 18-29 October
1 March 2010 Press Release Note No. 6244 Department of Public Information • News and Media Division • New York Note to Correspondents JAPANESE SINGER MISIA APPOINTED HONORARY AMBASSADOR FOR TENTH CONFERENCE OF PARTIES TO UN CONVENTION ON BIODIVERSITY, IN NAGOYA, 18-29 OCTOBER Japanese female singer MISIA has been appointed as an Honorary Ambassador for the tenth Meeting of the Conference of the Parties to the United Nations Convention on Biological Diversity, which will be held from 18 to 29 October 2010 in Nagoya, Aichi Prefecture, Japan. The Conference will focus on the post-2010 biodiversity targets by gathering opinions from diverse groups of stakeholders on what actions should be taken in the short- and medium-term, and how these actions will be implemented. Prior to this, and in support of the 2010 International Year of Biodiversity, a one-day High-Level Meeting on Biodiversity will bring together Heads of State, Government and delegations during the sixty-fifth session of the General Assembly in September in New York. These two meetings will shape the biodiversity agenda in the coming years and will need to address the challenges of accelerated biodiversity loss caused by overfishing, deforestation and pollution, and compounded by climate change. In her honorary role, MISIA will help raise awareness of the continuing depletion of biological resources and inform the public on the sustainable use of biodiversity. “In this International Year of Biodiversity, we must counter the perception that people are disconnected from our natural environment. We must increase understanding of the implications of losing biodiversity,” said Secretary-General Ban Ki-moon. -

(March 16, 2012) PUBLIC DOCUMENTS
PRESS RELEASE IOWA BOARD OF MEDICINE (March 16, 2012) PUBLIC DOCUMENTS: Please click on the physician’s name to access a link to a copy of the public documents for each physician. Recently, the Iowa Board of Medicine took the following actions: Douglas T. Cody, M.D., a 47-year-old Iowa-licensed physician who practices otolaryngology in Clinton, Iowa, had the terms of his probation terminated by the Board on March 9, 2012. On November 10, 2005, the Board charged Dr. Cody with engaging in professional incompetency and practice harmful or detrimental to the public. A hearing was held and on January 28, 2008, the Board issued a Final Decision. The Board concluded that Dr. Cody closed his medical practice without providing proper notice to patients, failed to provide appropriate treatment to patients and engaged in a pattern of disruptive behavior. Dr. Cody’s Iowa medical license was suspended, however the suspension was stayed and he was required to complete a Board-approved disruptive physician evaluation, submit a practice improvement plan and complete a Board-approved record keeping course. The Board issued Dr. Cody a public reprimand and ordered him to pay a $5,000 fine. The Board also placed Dr. Cody on probation subject to Board monitoring for a period of five years. Recently, Dr. Cody requested termination of the terms of his probation. On March 1, 2012, after careful consideration, the Board voted to terminate the terms of Dr. Cody’s probation. David K. Jones, M.D., a 45-year-old Iowa-licensed physician who practices internal medicine in Des Moines, Iowa, had the terms of his probation terminated by the Board on March 9, 2012. -

The Global Entrepreneurship Monitor (GEM) and Its Impact on Entrepreneurship Research Full Text Available At
Full text available at: http://dx.doi.org/10.1561/0300000033 The Global Entrepreneurship Monitor (GEM) and Its Impact on Entrepreneurship Research Full text available at: http://dx.doi.org/10.1561/0300000033 The Global Entrepreneurship Monitor (GEM) and Its Impact on Entrepreneurship Research Niels Bosma Utrecht University School of Economics & Global Entrepreneurship Research Association The Netherlands [email protected] Boston { Delft Full text available at: http://dx.doi.org/10.1561/0300000033 Foundations and Trends R in Entrepreneurship Published, sold and distributed by: now Publishers Inc. PO Box 1024 Hanover, MA 02339 USA Tel. +1-781-985-4510 www.nowpublishers.com [email protected] Outside North America: now Publishers Inc. PO Box 179 2600 AD Delft The Netherlands Tel. +31-6-51115274 The preferred citation for this publication is N. Bosma, The Global Entrepreneurship Monitor (GEM) and Its Impact on Entrepreneurship Research, Foundations and Trends R in Entrepreneurship, vol 9, no 2, pp 143{248, 2013. ISBN: 978-1-60198-634-4 c 2013 N. Bosma All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, mechanical, photocopying, recording or otherwise, without prior written permission of the publishers. Photocopying. In the USA: This journal is registered at the Copyright Clearance Cen- ter, Inc., 222 Rosewood Drive, Danvers, MA 01923. Authorization to photocopy items for internal or personal use, or the internal or personal use of specific clients, is granted by now Publishers Inc for users registered with the Copyright Clearance Center (CCC).