Partial Androgen Insensitivity Syndrome Presenting with Gynecomastia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypospadias by Ronald S
Kapi‘olani Pediatric Urology Hypospadias By Ronald S. Sutherland, M.D., F.A.A.P., F.A.C.S. What is hypospadias? Defined as a congenital deformity where the urethral opening is located beneath the penis rather then the tip, hypospadias ranges from a mild to severe deformity. The more common form is the distal variety, with the opening toward the front of the penis, which can usually be repaired with pleasing cosmetic results. More severe forms may be associated with the opening located at the base of the penis or further back near the anus. Usually hypospadias is also present with downward curvature of the penis (chordee), and a flattening of the foreskin with a hood-like covering. Occasionally the scrotum is also malformed and appears higher around the penis. Hypospadias can be found in a variety of congenital syndromes, including those with cardiac, renal, and testicular anomalies. (see next page) What causes hypospadias? Hypospadias develops early in gestation and occurs for unknown reasons, although there is slight familial tendency. Recent evidence suggests that the incidence is increasing and may be linked to environmental and genetic disruptions during the period when genital development is particularly sensitive to sex-steroid hormone imbalances. Occasionally, hypospadias can be detected by prenatal ultrasound. No pre-natal treatment or intervention is available. Parents should not request a circumcision for their newborn son with hypospadias because the foreskin may be necessary to assist with surgical repair. Sometimes hypospadias is not recognized until after the circumcision is completed. These cases are usually mild forms that can be repaired without the use of foreskin. -

Hypospadias Repair
Patient and Family Education Hypospadias Repair What causes it? What is hypospadias? Incomplete development of the urethra causes Hypospadias is a birth defect of the penis. hypospadias. It can occur in families. The The urethral opening, the hole where the urine comes out, is not in the normal position. reason for it happening is usually not known. Instead of the tip, it is on the undersurface of Why is it important to recognize the penis. Boys with hypospadias are usually hypospadias? missing the underside of their foreskin so An abnormally placed urethral opening does that the foreskin forms a hood. For this not allow the urine to pass as it s hould. A reason, most boys born with boy with hypospadias urinates with a hypospadias are not circumcised. There is stream that is often directed downward often a bend or curve (called chordee) in the rather than out and away from the body. This penis when the boy has an erection. causes wet pants and shoes. This condition, Hypospadias may be mild, moderate, or when left uncorrected, may make future severe depending on how far back the opening sexual intercourse difficult, or is and how much curvature is present. The impossible. more severe forms of hypospadias are usually associated with worsening degrees of chordee. Can it be corrected? Yes, surgery can correct the problem. These operations are best done between 6 and 18 months of age. The repair is usually performed in one surgery. If the hypospadias is severe, it may be necessary to have more than one surgery. The Normal position for urethra surgery usually lasts 1-3 hours and the patient goes home the same day. -

Background Briefing Document from the Consortium of Sponsors for The
BRIEFING BOOK FOR Pediatric Advisory Committee (PAC) Prepared by Alan Rogol, M.D., Ph.D. Prepared for Consortium of Sponsors Meeting Date: April 8th, 2019 TABLE OF CONTENTS LIST OF FIGURES ................................................... ERROR! BOOKMARK NOT DEFINED. LIST OF TABLES ...........................................................................................................................4 1. INTRODUCTION AND BACKGROUND FOR THE MEETING .............................6 1.1. INDICATION AND USAGE .......................................................................................6 2. SPONSOR CONSORTIUM PARTICIPANTS ............................................................6 2.1. TIMELINE FOR SPONSOR ENGAGEMENT FOR PEDIATRIC ADVISORY COMMITTEE (PAC): ............................................................................6 3. BACKGROUND AND RATIONALE .........................................................................7 3.1. INTRODUCTION ........................................................................................................7 3.2. PHYSICAL CHANGES OF PUBERTY ......................................................................7 3.2.1. Boys ..............................................................................................................................7 3.2.2. Growth and Pubertal Development ..............................................................................8 3.3. AGE AT ONSET OF PUBERTY.................................................................................9 3.4. -
Case Report Full Text Online At
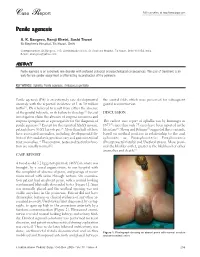
Case Report Full text online at http://www.jiaps.com Penile agenesis A. K. Bangroo, Ramji Khetri, Sashi Tiwari St Stephen's Hospital, Tis Hazari, Delhi Correspondence: AK Bangroo, 103, Administrative block, St. Stephens Hospital, Tis Hazari, Delhi-110054, India. E-mail: [email protected] ABSTRACT Penile agenesis is an extremely rare disorder with profound urological and psychological consequences. The goal of treatment is an early female gender assignment and feminizing reconstruction of the perineum. KEY WORDS: Aphallia, Penile agenesis, Ambiguous genitalia Penile agenesis (PA) is an extremely rare developmental the scrotal folds which were preserved for subsequent anomaly with the reported incidence of 1 in 30 million genital reconstruction. births[1]. PA is believed to result from either the absence of the genital tubercle, or its failure to develop.[2] Several DISCUSSION investigators claim the absence of corpora cavernosa and corpora spongiosum as a prerequisite for the diagnosis of The earliest case report of aphallia was by Imminger in penile agenesis.[3] Except for the reported XX-XY mosaic, 1853[2] since then only 75 cases have been reported in the patients have 46 XY karyotypes.[4] More than half of these literature[6]. Skoog and Belman[5] suggested three variants, have associated anomalies, including developmental de based on urethral position in relationship to the anal fects of the caudal axis, genitourinary and gastrointestinal sphincter, as: Postsphincteric; Presphincteric tract anomalies.[5] The scrotum, testes and testicular func (Prostatorectal fistula) and Urethral atresia. More proxi tion are usually normal[2]. mal the bladder outlet, greater is the likelihood of other anomalies and death.[5] CASE REPORT A two-day-old 3.2 kg genotypic male (46XY) neonate was brought, by a social organization, to our hospital with the complaint of absence of penis, and passage of meco nium mixed with urine through rectum. -

CASODEX (Bicalutamide)
HIGHLIGHTS OF PRESCRIBING INFORMATION • Gynecomastia and breast pain have been reported during treatment with These highlights do not include all the information needed to use CASODEX 150 mg when used as a single agent. (5.3) CASODEX® safely and effectively. See full prescribing information for • CASODEX is used in combination with an LHRH agonist. LHRH CASODEX. agonists have been shown to cause a reduction in glucose tolerance in CASODEX® (bicalutamide) tablet, for oral use males. Consideration should be given to monitoring blood glucose in Initial U.S. Approval: 1995 patients receiving CASODEX in combination with LHRH agonists. (5.4) -------------------------- RECENT MAJOR CHANGES -------------------------- • Monitoring Prostate Specific Antigen (PSA) is recommended. Evaluate Warnings and Precautions (5.2) 10/2017 for clinical progression if PSA increases. (5.5) --------------------------- INDICATIONS AND USAGE -------------------------- ------------------------------ ADVERSE REACTIONS ----------------------------- • CASODEX 50 mg is an androgen receptor inhibitor indicated for use in Adverse reactions that occurred in more than 10% of patients receiving combination therapy with a luteinizing hormone-releasing hormone CASODEX plus an LHRH-A were: hot flashes, pain (including general, back, (LHRH) analog for the treatment of Stage D2 metastatic carcinoma of pelvic and abdominal), asthenia, constipation, infection, nausea, peripheral the prostate. (1) edema, dyspnea, diarrhea, hematuria, nocturia, and anemia. (6.1) • CASODEX 150 mg daily is not approved for use alone or with other treatments. (1) To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca Pharmaceuticals LP at 1-800-236-9933 or FDA at 1-800-FDA-1088 or ---------------------- DOSAGE AND ADMINISTRATION ---------------------- www.fda.gov/medwatch The recommended dose for CASODEX therapy in combination with an LHRH analog is one 50 mg tablet once daily (morning or evening). -

EAU Pocket Guidelines on Male Hypogonadism 2013
GUIDELINES ON MALE HYPOGONADISM G.R. Dohle (chair), S. Arver, C. Bettocchi, S. Kliesch, M. Punab, W. de Ronde Introduction Male hypogonadism is a clinical syndrome caused by andro- gen deficiency. It may adversely affect multiple organ func- tions and quality of life. Androgens play a crucial role in the development and maintenance of male reproductive and sexual functions. Low levels of circulating androgens can cause disturbances in male sexual development, resulting in congenital abnormalities of the male reproductive tract. Later in life, this may cause reduced fertility, sexual dysfunc- tion, decreased muscle formation and bone mineralisation, disturbances of fat metabolism, and cognitive dysfunction. Testosterone levels decrease as a process of ageing: signs and symptoms caused by this decline can be considered a normal part of ageing. However, low testosterone levels are also associated with several chronic diseases, and sympto- matic patients may benefit from testosterone treatment. Androgen deficiency increases with age; an annual decline in circulating testosterone of 0.4-2.0% has been reported. In middle-aged men, the incidence was found to be 6%. It is more prevalent in older men, in men with obesity, those with co-morbidities, and in men with a poor health status. Aetiology and forms Male hypogonadism can be classified in 4 forms: 1. Primary forms caused by testicular insufficiency. 2. Secondary forms caused by hypothalamic-pituitary dysfunction. 164 Male Hypogonadism 3. Late onset hypogonadism. 4. Male hypogonadism due to androgen receptor insensitivity. The main causes of these different forms of hypogonadism are highlighted in Table 1. The type of hypogonadism has to be differentiated, as this has implications for patient evaluation and treatment and enables identification of patients with associated health problems. -

A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years
Journal of Advances in Radiology and Medical Imaging Volume 4 | Issue 1 ISSN: 2456-5504 Case Report Open Access A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man D’Amato D*, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, Valente F and Bizzaglia M Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Rome, Italy *Corresponding author: D’Amato D, Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Viale Oxford 181, Rome, Italy, Tel: +393207034690, E-mail: [email protected] Citation: D’Amato D, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, et al. (2019) A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man. J Adv Radiol Med Image 4(1): 102 Received Date: April 04, 2019 Accepted Date: August 26, 2019 Published Date: August 28, 2019 Abstract Congenital anorchia is an uncommon clinical condition. Etiology and pathogenetic mechanisms are often unknown. Although some patients with anorchia present with ambiguous external genitalia or micropenis, most have a normal phenotype. XY Disorders of Sex Development classifications are numerous and success rate in establishing a precise diagnosis is far lower than in XX karyotype. We report the case of a young man, with 46 XY karyotype showing various uro-genital abnormalities. A definitive diagnosis was not established due to the complex clinical presentation. Ultrasonography and Magnetic Resonance Imaging techniques were useful tools in the definition of uro-genital anomalies and gonadal development in this complex scenario. Keywords: Anorchia; Cryptorchidism; Urogenital Anomalies; DSD; MRI List of abbreviations: MRI: Magnetic Resonance Imaging; US: Ultrasonography; DSD: Disorders of Sex Development, FSH: Follicle- Stimulating Hormone; HCG: Human Chorionic Gonadotropin; LH: Luteinizing Hormone; AMH Antimüllerian Hormone; LHRH LH- Releasing Hormone; SD: Standard Deviation Introduction The disorders of sexual differentiation constitute a challenging area for both diagnostic and therapeutic impact. -

Battle of Sex Hormones in Genitalia Anomalies
COMMENTARY COMMENTARY Battle of sex hormones in genitalia anomalies Liang Maa,b,1 exposures to antiandrogen or estrogenic com- aDivision of Dermatology, Department of Medicine, Washington University School of pounds can lead to a range of penile anoma- Medicine, St. Louis, MO 63110; and bDepartment of Developmental Biology, Washington lies similar to human CPAs (2). However, University School of Medicine, St. Louis, MO 63110 how and when EDCs may influence normal external genitalia development is not very clear. In PNAS, Zheng et al. (3) use a state- To cope with their transition to a terrestrial anomalies (CPAs), including ambiguous gen- of-the-art conditional androgen receptor lifestyle, vertebrates had to extensively modify italia, hypospadias, chordee, and micropenis, (AR) knockout mouse model to show that their reproductive organs to facilitate repro- represent one of the most common birth de- disruption of androgen signaling at different duction on land (1). The mammalian penis fects, second only to congenital cardiac de- developmental stages can produce the full represents such a pinnacle in mammalian fects. Hypospadias is an arrest in urethra spectrum of CPAs. The authors go on to evolution, which enabled internal fertilization development, such that the urethra opening show that androgen and estrogen signaling and successful land invasion. There are two is located anywhere along the ventral side of antagonize each other during neonatal mouse phases of external genitalia development in the penile shaft instead of at the glans penis. i genital development and identify a signaling mammals: ( ) an ambisexual stage, in which Chordee is the abnormal bending of the penis, molecule, Indian hedgehog (Ihh), as a novel male and female embryos undergo the same resulting from tethering of urethral epithelium AR target required for penile masculinization. -

Evaluation of Additional Anomalies in Concomitance of Hypospadias And
Türkiye Çocuk Hastalıkları Dergisi 222 Özgün Araştırma Original Article Turkish Journal of Pediatric Disease Evaluation of Additional Anomalies in Concomitance of Hypospadias and Undescended Testes Hipospadias ve İnmemiş Testis Birlikteliğinde Ek Anomali Sıklığının Değerlendirilmesi Ufuk ATES, Gülnur GÖLLÜ, Nil YAŞAM TAŞTEKİN, Anar QURBANOV, Günay EKBERLİ, Meltem BİNGÖL KOLOĞLU, Emin AYDIN YAĞMURLU, Tanju AKTUĞ, Hüseyin DİNDAR, Ahmet Murat ÇAKMAK Ankara University Medical School, Pediatric Surgery Department, Pediatric Urology Division, Ankara, Turkey ABSTRACT Objective: Hypospadias is a common genitourinary system (GUS) anomaly in boys occurring in 1 of 200 to 300 live births. Undescended testes is frequently detected among accompanying anomalies in cases with hypospadias. Especially in proximal hypospadias and bilateral cases, this association may indicate sexual differentiation disorders. The aim of the study was to evaluate the togetherness of additional anomalies in hypospadiac children with undescended testes. Material and Methods: Between 2007 and 2016, data of 392 children who underwent surgery for hypospadias were evaluated retrospectively. Urethral meatus was present at scrotal and penoscrotal in 65 cases (16.6%) and glanular, coronal, subcoronal and midpenile in 327 cases (83.4%). The cases were divided into two groups as those with both testes in the scrotum and those with undescended testes, and the anomalies were recorded. Results: The mean age of the children with proximal hypospadias was 21 months (6-240 months). Of the children with proximal hypospadias, 26 (40%) had undescended testes and 39 (60%) had testes in the scrotum. Undescended testes were detected bilaterally in 17 patients (65.4%) and unilaterally in nine patients (34.6%) in the undescended testes group. -

Growth Hormone Deficiency Causing Micropenis:Peter A
Growth Hormone Deficiency Causing Micropenis:Peter A. Lee, MD, PhD, a Tom Mazur, PsyD, Lessons b Christopher P. Houk, MD, Learned c Robert M. Blizzard, MD d From a Well-Adjusted Adultabstract This report of a 46, XY patient born with a micropenis consistent with etiology from isolated congenital growth hormone deficiency is used to (1) raise the question regarding what degree testicular testosterone exposure aDepartment of Pediatrics, College of Medicine, Penn State to the central nervous system during fetal life and early infancy has on the University, Hershey, Pennsylvania; bCenter for Psychosexual development of male gender identity, regardless of gender of rearing; (2) Health, Jacobs School of Medicine and Biomedical suggest the obligatory nature of timely full disclosure of medical history; Sciences, University at Buffalo and John R. Oishei Children’s Hospital, Buffalo, New York; cDepartment of (3) emphasize that virtually all 46, XY infants with functional testes and Pediatrics, Medical College of Georgia, Augusta University, a micropenis should be initially boys except some with partial androgen Augusta, Georgia; and dDepartment of Pediatrics, College of Medicine, University of Virginia, Charlottesville, Virginia insensitivity syndrome; and (4) highlight the sustaining value of a positive long-term relationship with a trusted physician (R.M.B.). When this infant Dr Lee reviewed and discussed the extensive medical records with Dr Blizzard, reviewed presented, it was commonly considered inappropriate to gender assign an pertinent medical literature, and wrote each draft infant male whose penis was so small that an adult size was expected to be of the manuscript with input from all coauthors; inadequate, even if the karyotype was 46, XY, and testes were functional. -

Gynecomastia-Like Hyperplasia of Female Breast
Case Report Annals of Infertility & Reproductive Endocrinology Published: 25 May, 2018 Gynecomastia-Like Hyperplasia of Female Breast Haitham A Torky1*, Anwar A El-Shenawy2 and Ahmed N Eesa3 1Department of Obstetrics-Gynecology, As-Salam International Hospital, Egypt 2Department of Surgical Oncology, As-Salam International Hospital, Egypt 3Department of Pathology, As-Salam International Hospital, Egypt Abstract Introduction: Gynecomastia is defined as abnormal enlargement in the male breast; however, histo-pathologic abnormalities may theoretically occur in female breasts. Case: A 37 years old woman para 2 presented with a right painless breast lump. Bilateral mammographic study revealed right upper quadrant breast mass BIRADS 4b. Wide local excision of the mass pathology revealed fibrocystic disease with focal gynecomastoid hyperplasia. Conclusion: Gynecomastia-like hyperplasia of female breast is a rare entity that resembles malignant lesions clinically and radiological and is only distinguished by careful pathological examination. Keywords: Breast mass; Surgery; Female gynecomastia Introduction Gynecomastia is defined as abnormal enlargement in the male breast; however, the histo- pathologic abnormalities may theoretically occur in female breasts [1]. Rosen [2] was the first to describe the term “gynecomastia-like hyperplasia” as an extremely rare proliferative lesion of the female breast which cannot be distinguished from florid gynecomastia. The aim of the current case is to report one of the rare breast lesions, which is gynecomastia-like hyperplasia in female breast. Case Presentation A 37 years old woman para 2 presented with a right painless breast lump, which was accidentally OPEN ACCESS discovered 3 months ago and of stationary course. There was no history of trauma, constitutional symptoms or nipple discharge. -
Micropenis Associated with Testicular Agenesis
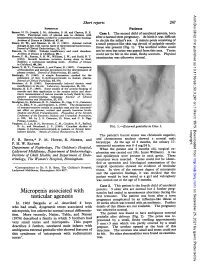
Arch Dis Child: first published as 10.1136/adc.50.3.247 on 1 March 1975. Downloaded from Short reports 247 REFERENCES Patients Barnes, N. D., Joseph, J. M., Atherden, S. M. and Clayton, B. E. (1972). Functional tests of adrenal axis in children with Case 1. The second child of unrelated parents, born measurement of plasma cortisol by competitive protein binding. after a normal term pregnancy. At birth it was difficult Archives of Disease in Childhood, 47, 66. to decide the infant's sex. A minute penis consisting of Deane, H. W., and Masson, G. M. C. (1951). Adrenal cortical a small prepuce-like skin tag devoid of palpable erectile changes in rats with various types of experimental hypertension. .ournal of Clinical Endocrinology, 11, 193. tissue was present (Fig. 1). The urethral orifice could Fanconi, G. (1954). Tubular insufficiency and renal dwarfism. not be seen but urine was passed from this area. Testes Archives of Disease in Childhood, 29, 1. could not be felt in the small, fleshy scrotum. Physical Howse, P. M., Rayner, P. H. W., Williams, J. W., and Rudd, B. T. (1974). Growth hormone secretion during sleep in short examination was otherwise normal. children: a continuous sampling study. Archives of Disease in Childhood, 49, 246. James, V. H. T., Townsend, J., and Fraser, R. (1967). Comparison of fluorimetric and isotopic procedures for the determination of plasma cortisol. journal of Endocrinology, 37, xxviii. Mattingly, D. (1962). A simple fluorimetric method for the estimation of free 1 1-hydroxycorticoids in human plasma. Journal of Clinical Pathology, 15, 374.