The Golden Jackal Canis Aureus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevalence of Intestinal Helminth Infections in Dogs and Two Species of Wild Animals from Samarkand Region of Uzbekistan
ISSN (Print) 0023-4001 ISSN (Online) 1738-0006 Korean J Parasitol Vol. 57, No. 5: 549-552, October 2019 ▣ BRIEF COMMUNICATION https://doi.org/10.3347/kjp.2019.57.5.549 Prevalence of Intestinal Helminth Infections in Dogs and Two Species of Wild Animals from Samarkand Region of Uzbekistan Tai-Soon Yong1, Kyu-Jae Lee2, Myeong Heon Shin1, Hak Sun Yu3, Uktamjon Suvonkulov4, 4 5 6, Turycin Bladimir Sergeevich , Azamat Shamsiev , Gab-Man Park * 1Department of Environmental Medical Biology and Institute of Tropical Medicine, Yonsei University College of Medicine, Seoul 03722, Korea; 2Department of Environmental Medical Biology, Yonsei University Wonju College of Medicine, Wonju 26426, Korea; 3Department of Parasitology and Tropical Medicine, School of Medicine, Pusan National University, Yangsan 50612, Korea; 4Isaev Research Institute of Medical Parasitology, Ministry of Health, Samarkand, Republic of Uzbekistan; 5Department of Pediatric Surgery, Samarkand Medical Institute, Samarkand, Republic of Uzbekistan; 6Department of Environmental Medical Biology, Catholic Kwandong University College of Medicine, Gangneung 25601, Korea Abstract: This study aimed to determine the prevalence of intestinal helminth parasitic infections and associated risk fac- tors for the human infection among the people of Samarkand, Uzbekistan. Infection status of helminths including Echino- coccus granulosus was surveyed in domestic and wild animals from 4 sites in the Samarkand region, Uzbekistan during 2015-2018. Fecal samples of each animal were examined with the formalin-ether sedimentation technique and the recov- ery of intestinal helminths was performed with naked eyes and a stereomicroscope in total 1,761 animals (1,755 dogs, 1 golden jackal, and 5 Corsac foxes). Total 658 adult worms of E. -

Efficacy of Simparica Trio™, a Novel Chewable Tablet Containing
Becskei et al. Parasites Vectors (2020) 13:99 https://doi.org/10.1186/s13071-020-3951-4 Parasites & Vectors RESEARCH Open Access Efcacy of Simparica Trio™, a novel chewable tablet containing sarolaner, moxidectin and pyrantel, against induced hookworm infections in dogs Csilla Becskei1*, Mirjan Thys1, Kristina Kryda2, Leon Meyer3,4, Susanna Martorell5, Thomas Geurden1, Leentje Dreesen1, Tiago Fernandes1 and Sean P. Mahabir2 Abstract Background: Ancylostomatids (‘hookworms’) are among the most important zoonotic nematode parasites infecting dogs worldwide. Ancylostoma caninum and Uncinaria stenocephala are two of the most common hookworm species that infect dogs. Both immature and adult stages of hookworms are voracious blood feeders and can cause death in young dogs before infection can be detected by routine fecal examination. Hence, treatment of both immature and adult stages of hookworms will decrease the risk of important clinical disease in the dog as well as the environmental contamination caused by egg-laying adults, which should reduce the risk of infection for both dogs and humans. The studies presented here were conducted to evaluate the efcacy of a novel, oral chewable tablet containing sarolaner, ™ moxidectin and pyrantel (Simparica Trio ), against induced larval (L4), immature adult (L5) and adult A. caninum, and adult U. stenocephala infections in dogs. Methods: Eight negative-controlled, masked, randomized laboratory studies were conducted. Two separate studies were conducted against each of the target parasites and stages. Sixteen or 18 purpose bred dogs, 8 or 9 in each of the two treatment groups, were included in each study. Dogs experimentally infected with the target parasite were dosed once on Day 0 with either placebo tablets or Simparica Trio™ tablets to provide minimum dosages of 1.2 mg/kg sarolaner, 24 µg/kg moxidectin and 5.0 mg/kg pyrantel (as pamoate salt). -

Managing Hookworms in the Landscape 1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. ENY-017 Managing Hookworms in the Landscape 1 Robert A. Dunn and Ellis C. Greiner2 "Hookworms" properly refers to many genera of • A. tubaeforme is the common hookworm of nematodes in the Family Ancylostomatidae of the cats, distributed world-wide; similar to A. Order Strongylida, but this discussion addresses caninum, but generally smaller. primarily those in the genus Ancylostoma, which many animal health professionals consider to be the • Uncinaria stenocephala occurs in the small most important genus of hookworms. This genus intestine of dogs, cats, foxes, wolves, and related includes the most common hookworms of domestic carnivores. It is occasionally recovered from dogs and cats in tropical and warm temperate stray dogs in Florida, but may not occur climates, the hookworms with which most people in endemically here -- the infections that are Florida come into contact. The "northern carnivore detected may have occurred farther north, before hookworm," Uncinaria stenocephala, also occurs in the host animals came to Florida. Florida but much less frequently than Ancylostoma Importance as Animal and Human spp. Parasites Four species are significant in Florida; the three Ancylostoma spp. represent 90 - 95% of hookworms Widely distributed wherever dogs and cats are identified here: kept as pets, hookworms are found commonly in the small intestines of hosts in which they can complete • Ancylostoma caninum, the dog hookworm, is their life cycles. Hookworms suck blood from the found in the small intestine of dogs, foxes, intestinal wall. The degree of blood sucking varies coyotes, wolves, bears, and other wild carnivores among these hookworms. -

Prevalence of Intestinal Nematodes of Red Foxes (Vulpes Vulpes) in North-West Poland
Prevalence of intestinal nematodes of red foxes (Vulpes vulpes) in north-west Poland Agnieszka Tylkowska ( [email protected] ) Szkola Glowna Gospodarstwa Wiejskiego https://orcid.org/0000-0002-7406-7094 Bogumiła Pilarczyk Zachodniopomorski Uniwersytet Technologiczny w Szczecinie Agnieszka Tomza-Marciniak Zachodniopomorski Uniwersytet Technologiczny w Szczecinie Renata Pilarczyk Zachodniopomorski Uniwersytet Technologiczny w Szczecinie Research Keywords: red fox, prevalence, helminths, nematodes, ecological indicators Posted Date: April 9th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-21823/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/11 Abstract Background: The red fox (Vulpes vulpes) is a widely distributed animal in the world. This wild carnivore is also a common host of several dangerous zoonotic parasites, primarily nematodes. Nematodes of red foxes, such as Toxocara canis and Uncinaria stenocephala, can cause numerous health problems in humans and domesticated animals. The aim of the study was to determine the parameters of occurrence of nematodes in red fox (Vulpes vulpes) in north-western Poland. Methods: The study was carried out in north-western Poland. The research material consisted of 620 red foxes (Vulpes vulpes). Parasitological sections of the foxes were taken using the sedimentation and counting technique. Results: The prevalence of infestations with nematodes was 77.3%, while the mean infection intensity was 20.1 per animal. The presence of Toxocara canis, Toxascaris leonina, Uncinaria stenocephala and Trichuris vulpis was noted. The greatest prevalence was presented by Uncinaria stenocephala (34.0%). Male and female foxes displayed a similar prevalence of nematodes. Their presence was recorded in the duodenum, jejunum, ileum and caecum of the foxes, and they were signicantly more common in the jejunum than in other parts. -

The Prevalence of Intestinal Nematodes Among Red Foxes
Tylkowska et al. Acta Vet Scand (2021) 63:19 https://doi.org/10.1186/s13028-021-00584-0 Acta Veterinaria Scandinavica RESEARCH Open Access The prevalence of intestinal nematodes among red foxes (Vulpes vulpes) in north-western Poland Agnieszka Tylkowska1* , Bogumiła Pilarczyk2, Agnieszka Tomza‑Marciniak2 and Renata Pilarczyk3 Abstract Background: The red fox (Vulpes vulpes) is widely distributed in the Northern Hemisphere and Australia. The pres‑ ence of nematode‑infected foxes in urbanized areas increases the risk of transmission of nematodes to domestic dogs and thus, to humans. The aim of this study was to determine the prevalence and species composition of intestinal nematodiasis in red foxes in Western Pomerania, a province in north‑western Poland. The intestinal contents of 620 red foxes killed during a government reduction shooting programme were examined for adult nematodes using the sedimentation and counting technique (SCT). Results: Intestinal nematodes, including Toxocara canis, Toxascaris leonina, Uncinaria stenocephala and Trichuris vulpis, were found in 77.3% (95% CI 73.8–80.4%) of the examined foxes with a mean infection burden of 20.1 nematode per animal. Male and female foxes had similar infection burdens. Conclusions: The nematodes are present in high prevalence and intensity among foxes in north‑western Poland. Furthermore, this high prevalence of nematodes in foxes may likely constitute a health risk to humans and domestic animals due to increasing fox densities in urban and periurban areas. Keywords: Ecological indicators, Helminths, Nematodes, Prevalence, Red fox Background in urbanized areas increases the risk of transmission of Te red fox (Vulpes vulpes) is widely distributed in the these nematodes to humans, either directly, or indirectly Northern Hemisphere and Australia [1]. -

Zoonotic Nematodes of Wild Carnivores
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 Zoonotic nematodes of wild carnivores Otranto, Domenico ; Deplazes, Peter Abstract: For a long time, wildlife carnivores have been disregarded for their potential in transmitting zoonotic nematodes. However, human activities and politics (e.g., fragmentation of the environment, land use, recycling in urban settings) have consistently favoured the encroachment of urban areas upon wild environments, ultimately causing alteration of many ecosystems with changes in the composition of the wild fauna and destruction of boundaries between domestic and wild environments. Therefore, the exchange of parasites from wild to domestic carnivores and vice versa have enhanced the public health relevance of wild carnivores and their potential impact in the epidemiology of many zoonotic parasitic diseases. The risk of transmission of zoonotic nematodes from wild carnivores to humans via food, water and soil (e.g., genera Ancylostoma, Baylisascaris, Capillaria, Uncinaria, Strongyloides, Toxocara, Trichinella) or arthropod vectors (e.g., genera Dirofilaria spp., Onchocerca spp., Thelazia spp.) and the emergence, re-emergence or the decreasing trend of selected infections is herein discussed. In addition, the reasons for limited scientific information about some parasites of zoonotic concern have been examined. A correct compromise between conservation of wild carnivores and risk of introduction and spreading of parasites of public health concern is discussed in order to adequately manage the risk of zoonotic nematodes of wild carnivores in line with the ’One Health’ approach. DOI: https://doi.org/10.1016/j.ijppaw.2018.12.011 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-175913 Journal Article Published Version The following work is licensed under a Creative Commons: Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) License. -

Cutaneous Larva Migrans: the Creeping Eruption
Cutaneous Larva Migrans: The Creeping Eruption Marc A. Brenner, DPM; Mital B. Patel, DPM Cutaneous larva migrans (CLM) is the most com- Hospital in Ontario, Canada, 48% of patients with mon tropically acquired dermatosis. It is caused CLM had recently traveled to Jamaica.2 CLM is an by hookworm larvae, which are in the feces of animal hookworm infestation usually caused by the infected dogs and cats. The condition occurs Ancylostoma genus of nematodes. It is confined pre- mainly in the Caribbean and New World, and dominantly to tropical and subtropical countries, anyone walking barefoot or sitting on a contami- although its distribution is ubiquitous. Eggs of the nated beach is at risk. nematode (usually Ancylostoma braziliense) are Ancylostoma braziliense and Ancylostoma found most commonly in dog and cat feces. In caninum are the most common hookworms Uruguay, 96% of dogs are infected by hookworms.3 responsible for CLM. The lesions, called creep- An individual is exposed to the larvae by sitting or ing eruptions, are characteristically erythema- walking on a beach that has been contaminated tous, raised and vesicular, linear or serpentine, with dog or cat feces. In a retrospective survey of and intensely pruritic. The conditions respond to 44 cases of CLM presented at the Hospital for oral and/or topical application of thiabendazole. Tropical Diseases in London, 95% of patients Humans become an accidental dead-end host reported a history of exposure at a beach.4 Activi- because the traveling parasite perishes, and its ties that pose a risk include contact with contami- cutaneous manifestations usually resolve nated sand or soil, such as playing in a sandbox, uneventfully within months. -
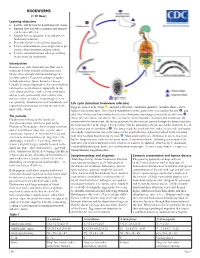
HOOKWORMS (1 CE Hour) Learning Objectives !! List the Risk Factors for Hookworm Infections
HOOKWORMS (1 CE Hour) Learning objectives ! List the risk factors for hookworm infections. ! Explain how and where humans and animals can become infected. ! Explain how to diagnose, treat and prevent hookworm infection. ! Describe the life cycle of these parasites. ! List recommendations you can provide to pet owners about treatment and prevention. ! List the contraindications when prescribing medications for hookworm. Introduction Zoonoses are infectious diseases that can be transmitted from animals to humans, most likely when animals and human beings are in close contact. Causative groups of agents include parasites, fungi, bacteria, viruses. Usually of minor importance, they nevertheless can lead to severe disease, especially in the very young and those with certain conditions, and to death, particularly with certain viral diseases (such as rabies, hemorrhagic fevers, encephalitis). Hookworms exist worldwide and Life cycle (Intestinal hookworm infection) cross from carnivorous animals to man in all Eggs are passed in the stool , and under favorable conditions (moisture, warmth, shade), larvae parts of the world. hatch in one to two days. The released rhabditiform larvae grow in the feces and/or the soil , and The parasite after 5 to 10 days (and two molts) they become filariform (third-stage) larvae that are infective . These infective larvae can survive three to four weeks in favorable environmental conditions. On Hookworms belong to the family of contact with the human host, the larvae penetrate the skin and are carried through the blood vessels to Ancylostomatidae which are part of the the heart and then to the lungs. They penetrate into the pulmonary alveoli, ascend the bronchial tree to Phylum of Nematodes: Ancylostoma caninum the pharynx and are swallowed . -

A Parasitological Survey of the Cascade Red Fox
A PARASITOLOGICAL SURVEY OF THE CASCADE RED FOX (VULPES VULPES CASCADENSIS) AND THE COYOTE (CANIS LATRANS) IN MOUNT RAINIER NATIONAL PARK by Jessica Brown A Thesis Submitted in partial fulfillment of the requirements for the degree Master of Environmental Studies The Evergreen State College June 2018 ©2018 by Jessica Brown. All rights reserved. This Thesis for the Master of Environmental Studies Degree by Jessica Brown has been approved for The Evergreen State College by ________________________ Tara Chestnut, PhD Member of the Faculty ________________________ Date ABSTRACT A parasitological survey of the Cascade red fox (Vulpes vulpes cascadensis) and the coyote (Canis latrans) in Mount Rainier National Park. Jessica Brown Loss of biodiversity is widespread and increasing numbers of carnivores in North America are suffering from population decline and reduced distribution. The risk of extinction is reality for many of these species, predominately due to the consequences of human activities. The complexity of biodiversity loss has been linked to environmental alterations such as habitat loss and fragmentation, pollution, urbanization, and climate change. In addition, disease emergence among wildlife, including parasitism, is accelerating at an alarming rate. Parasites and pathogens often interact with other environmental stressors and cause species population decline. Species with small populations and low genetic diversity are at the greatest risk of extirpation. Thus the aim of this study was to identify parasitic helminths of the Cascade red fox (Vulpus vulpes cascadensis) and the sympatric coyote (Canis latrans) in the Mount Rainier National Park (MORA) of Washington State. Cascade red fox, an extremely rare mesocarnivore, has experienced a decline in population and a recent loss of genetic diversity. -

New Approachment of Creeping Eruption Management Sukmawati Tansil Tan* and Yohanes Firmansyah
ISSN: 2469-5750 Tan and Firmansyah. J Dermatol Res Ther 2020, 6:088 DOI: 10.23937/2469-5750/1510088 Volume 6 | Issue 2 Journal of Open Access Dermatology Research and Therapy CaSe RepoRT New Approachment of Creeping Eruption Management Sukmawati Tansil Tan* and Yohanes Firmansyah Check for Department of Dermato-Venereology, Tarumanagara University, Indonesia updates *Corresponding author: Sukmawati Tansil Tan, Department of Dermato-Venereology, Tarumanagara University, Indo- nesia cats [3]. Clinically, cutaneous larval migrans (CLM) are Abstract characterized by tortuous erythematous pruritic lesions Cutaneous larva migrans (CLM) is a zoonotic infestation or serpiginosa with slightly raised or prominent path- caused by penetration and migration of filariform larvae into the epidermal layer of skin derived from dogs and cats, ways. This disease was first introduced by Lee, a British namely Ancylostoma braziliense and Ancylostoma cani- doctor, in 1874. The term "cutaneous migratory larvae" num. Infective filariform larvae penetrate the surface of the was coined by Crocker in 1893, and in 1929 the etiolo- skin, and migrate beneath the epidermis by leaving promi- gy of this disease was known as Ancylostoma larvae, so nent linear or serpiginous lesions called 'creeping eruption'. One case was reported of CLM with the main complaint of the terminology cutaneous larva migrans (CLM) known being very itchy and serpiginosa lesion with hyperemic pap- as Hookworm-related cutaneous larva migrans (HrCLM) ules. Treatment with pyrantel pamoate and mebendazole [4]. is not effective in this case. Patients were given alternative therapies using 5% permethrin cream for 3 days. On the During these years, the terms HrCLM and creeping sixth day after 5% permethrin therapy, the lesion underwent eruption are considered to have the same meaning, total resolution. -

Worm Control in Dogs and Cats
Worm Control 1 in Dogs and Cats ESCCAP Guideline 01 Sixth Edition – May 2021 1 ESCCAP Malvern Hills Science Park, Geraldine Road, Malvern, Worcestershire, WR14 3SZ, United Kingdom First Edition Published by ESCCAP in December 2006 © ESCCAP 2006–2021 All rights reserved This publication is made available subject to the condition that any redistribution or reproduction of part or all of the contents in any form or by any means, electronic, mechanical, photocopying, recording or otherwise is with the prior written permission of ESCCAP. This publication may only be distributed in the covers in which it is first published unless with the prior written permission of ESCCAP. A catalogue record for this publication is available from the British Library. ISBN: 978-1-913757-18-2 2 TABLE OF CONTENTS INTRODUCTION 6 SCOPE 7 PRESENT SITUATION AND EMERGING THREATS 7 LIFELONG CONTROL OF COMMON WORMS 7 BIOLOGY, DIAGNOSIS AND CONTROL OF WORMS 11 1. Roundworms (Toxocara spp.) 11 2. Tapeworms 13 Echinococcus granulosus and Echinococcus multilocularis 13 Dipylidium caninum 16 Taenia spp. 17 3. Heartworm and Subcutaneous Worms 19 Dirofilaria immitis 19 Dirofilaria repens 20 Zoonotic potential of D. immitis and D. repens 21 4. French Heartworm (Angiostrongylus vasorum) 22 5. Hookworms (Ancylostoma spp. and Uncinaria spp.) 23 6. Whipworm (Trichuris vulpis) 24 DIAGNOSIS OF HELMINTH INFECTIONS 25 IMPACT OF PET HEALTH AND LIFESTYLE FACTORS 26 RESISTANCE TO ANTHELMINTICS 26 ENVIRONMENTAL CONTROL OF PARASITE TRANSMISSION 27 OWNER CONSIDERATIONS IN PREVENTING ZOONOTIC -

Helminth Infections in Domestic Dogs from Russia
Veterinary World, EISSN: 2231-0916 REVIEW ARTICLE Available at www.veterinaryworld.org/Vol.9/November-2016/14.pdf Open Access Helminth infections in domestic dogs from Russia T. V. Moskvina1 and A. V. Ermolenko2 1. Department of Biodiversity and Marine Bioresources, Far Eastern Federal University, School of Natural Sciences, 690922 Vladivostok, Russia; 2. Department of Zoological, Laboratory of Parasitology, Institute of Biology and Soil Science, Far-Eastern Branch of Russian Academy of Sciences, 690022 Vladivostok, Russia. Corresponding author: T. V. Moskvina, e-mail: [email protected], AVE: [email protected] Received: 17-07-2016, Accepted: 04-10-2016, Published online: 15-11-2016 doi: 10.14202/vetworld.2016.1248-1258 How to cite this article: Moskvina TV, Ermolenko AV (2016) Helminth infections in domestic dogs from Russia, Veterinary World, 9(11): 1248-1258. Abstract Dogs are the hosts for a wide helminth spectrum including tapeworms, flatworms, and nematodes. These parasites affect the dog health and cause morbidity and mortality, especially in young and old animals. Some species, as Toxocara canis, Ancylostoma caninum, Dipylidium caninum, and Echinococcus spp. are well-known zoonotic parasites worldwide, resulting in high public health risks. Poor data about canine helminth species and prevalence are available in Russia, mainly due to the absence of official guidelines for the control of dog parasites. Moreover, the consequent low quality of veterinary monitoring and use of preventive measures, the high rate of environmental contamination by dog feces and the increase of stray dog populations, make the control of the environmental contamination by dog helminths very difficult in this country. This paper reviews the knowledge on canine helminth fauna and prevalence in Russia.