Uncomplicated Monochorionic Twins: Two Normal Hearts Sharing One Placenta
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Association Between Chorionicity and Preterm Birth in Twin Pregnancies: a Systematic Review Involving 29 864 Twin Pregnancies
DOI: 10.1111/1471-0528.16479 Systematic Review www.bjog.org Association between chorionicity and preterm birth in twin pregnancies: a systematic review involving 29 864 twin pregnancies S Marleen,a,b C Dias,b R Nandasena,b R MacGregor,c J Allotey,d J Aquilina,c A Khalil,e,f S Thangaratinamg a Barts Research Centre for Women’s Health (BARC), Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK b Sri Jayewardenepura Postgraduate Teaching Hospital, Nugegoda, Sri Lanka c Royal London Hospital, Barts Health NHS Trust, London, UK d Institute of Applied Health Research, University of Birmingham, Birmingham, UK e St George’s University Hospitals NHS Foundation Trust, London, UK f Molecular and Clinical Sciences Research Institute, St George’s Medical School, University of London, London, UK g World Health Organization (WHO) Collaborating Centre for Global Women’s Health, Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, UK Correspondence: S Marleen, Barts Research Centre for Women’s Health (BARC), Barts and the London School of Medicine and Dentistry, Queen Mary University of London, Mile End Road, London E1 4NS, UK. Email: [email protected] Accepted 7 August 2020. Published Online 7 October 2020. Background The perinatal mortality and morbidity among twins I2 = 46%, OR 1.55, 95% CI 1.27–1.89 I2 = 68%, OR 1.47, 95% CI vary by chorionicity. Although it is considered that 1.27–1.69, I2 = 60%, OR 1.66, 95% CI 1.43–1.93, I2 = 65%, monochorionicity is associated with an increased risk of preterm respectively). -

Pre-Eclampsia/Eclampsia in Twin Pregnancies A
J Med Genet: first published as 10.1136/jmg.13.3.208 on 1 June 1976. Downloaded from Journal of Medical Genetics (1976). 13, 208-211. Pre-eclampsia/eclampsia in twin pregnancies A. McFARLANE and J. S. SCOTT From the Department of Obstetrics and Gynaecology (Leeds Maternity Hospital), University of Leeds, 17 Springfield Mount, Leeds LS2 9NG Summary. A study of 1045 twin gestations with regard to known or likely zygosity and the incidence of pre-eclampsia/eclampsia failed to reveal differences between known dizygous twins and like-sex 'presumed' and 'estimated' monozygous twins except in the 'estimated' data for multigravidae. There was a threefold in- crease in the incidence for twins as opposed to singleton pregnancies. These results are discussed in relation to increased conceptus-mother antigenic differences. It is suggested that the risk of gestosis in twin pregnancy involves more than a summa- tion ofthat operating in two singleton pregnancies. It has been suggested that genetic incompatibility Pregnancies were classified according to definitions given between mother and fetus may be a factor in the below. aetiology of pre-eclampsia (Penrose, 1946; Kalmus, 1946; Platt, Stewart, and Emery, 1958). Epidemio- A. Hypertension status logical evidence has been provided by Stevenson et (1) Mildpre-eclampsia-a basal blood pressure of 120/ 80 mm Hg or less recorded before 24 weeks' gestation, al (1971) in a study of consanguineous marriages in followed by a rise to 140/90 mm Hg or more on at least the Middle East. They also recorded twin data two occasions recorded in the antenatal ward, clinic which pointed to a higher incidence of toxaemia in readings being discounted, together with oedema and unlike-sex as opposed to like-sex twin pregnancies. -

A Case Report
Case Reports in Women's Health 19 (2018) e00073 Contents lists available at ScienceDirect Case Reports in Women's Health journal homepage: www.elsevier.com/locate/crwh Hyperreactio luteinalis in a monochorionic twin pregnancy complicated by preeclampsia: A case report Laura Sienas a,⁎, Trevor Miller b, Juliana Melo c, Herman Hedriana c a Department of Obstetrics and Gynecology, University of Washington, 1959 NE Pacific Street, Box 356460, Seattle, WA 98195, USA b Department of Obstetrics and Gynecology, Kaiser Permanente San Leandro, 2500 Merced St, San Leandro, CA 94577, USA c Department of Obstetrics and Gynecology, University of California Davis, 2315 Stockton Blvd, Sacramento, CA 95817, USA article info abstract Article history: Hyperreactio luteinalis (HL) is a rare benign complication of pregnancy that is characterized by progressive ovar- Received 9 June 2018 ian enlargement and hyperandrogenism. We present a case of a 30-year-old woman with a spontaneous Received in revised form 3 August 2018 monochorionic diamniotic twin pregnancy who presented with early-onset preeclampsia, concern about possi- Accepted 8 August 2018 ble twin-twin transfusion syndrome, and bilateral enlarged ovarian masses. Both ovaries had multiple thin- Available online xxxx walled unilocular cysts; one ovary measured 17.9 × 17.5 × 9.1 cm and the other 12.5 × 11 × 12.3 cm. After ex- tensive counseling, the patient underwent an uncomplicated dilation and evacuation. Postoperative assessment Keywords: Adnexal mass indicated elevated androgen levels, which spontaneously resolved, supporting the clinical diagnosis of HL. It is Pregnancy important to consider HL in the differential diagnosis of adnexal masses in pregnancy. HL spontaneously re- Hyperandrogenism gresses after delivery and is managed expectantly. -

OBGYN-Study-Guide-1.Pdf
OBSTETRICS PREGNANCY Physiology of Pregnancy: • CO input increases 30-50% (max 20-24 weeks) (mostly due to increase in stroke volume) • SVR anD arterial bp Decreases (likely due to increase in progesterone) o decrease in systolic blood pressure of 5 to 10 mm Hg and in diastolic blood pressure of 10 to 15 mm Hg that nadirs at week 24. • Increase tiDal volume 30-40% and total lung capacity decrease by 5% due to diaphragm • IncreaseD reD blooD cell mass • GI: nausea – due to elevations in estrogen, progesterone, hCG (resolve by 14-16 weeks) • Stomach – prolonged gastric emptying times and decreased GE sphincter tone à reflux • Kidneys increase in size anD ureters dilate during pregnancy à increaseD pyelonephritis • GFR increases by 50% in early pregnancy anD is maintaineD, RAAS increases = increase alDosterone, but no increaseD soDium bc GFR is also increaseD • RBC volume increases by 20-30%, plasma volume increases by 50% à decreased crit (dilutional anemia) • Labor can cause WBC to rise over 20 million • Pregnancy = hypercoagulable state (increase in fibrinogen anD factors VII-X); clotting and bleeding times do not change • Pregnancy = hyperestrogenic state • hCG double 48 hours during early pregnancy and reach peak at 10-12 weeks, decline to reach stead stage after week 15 • placenta produces hCG which maintains corpus luteum in early pregnancy • corpus luteum produces progesterone which maintains enDometrium • increaseD prolactin during pregnancy • elevation in T3 and T4, slight Decrease in TSH early on, but overall euthyroiD state • linea nigra, perineum, anD face skin (melasma) changes • increase carpal tunnel (median nerve compression) • increased caloric need 300cal/day during pregnancy and 500 during breastfeeding • shoulD gain 20-30 lb • increaseD caloric requirements: protein, iron, folate, calcium, other vitamins anD minerals Testing: In a patient with irregular menstrual cycles or unknown date of last menstruation, the last Date of intercourse shoulD be useD as the marker for repeating a urine pregnancy test. -

Risk Factors for Early and Late Onset Preeclampsia in Reunion Island: Multivariate Analysis of Singleton and Twin Pregnancies
reproductive medicine Article Risk Factors for Early and Late Onset Preeclampsia in Reunion Island: Multivariate Analysis of Singleton and Twin Pregnancies. A 20-Year Population-Based Cohort of 2120 Preeclampsia Cases Pierre-Yves Robillard 1,2,* , Malik Boukerrou 2,3, Gustaaf Dekker 4, Marco Scioscia 5 , Francesco Bonsante 1,2, Brahim Boumahni 1 and Silvia Iacobelli 1,2 1 Service de Néonatologie, Centre Hospitalier Universitaire Sud Réunion, BP 350, CEDEX, 97448 Saint-Pierre, La Réunion, France; [email protected] (F.B.); [email protected] (B.B.); [email protected] (S.I.) 2 Centre d’Etudes Périnatales Océan Indien (CEPOI), Centre Hospitalier Universitaire Sud Réunion, BP 350, CEDEX, 97448 Saint-Pierre, La Reunion, France; [email protected] 3 Service de Gynécologie et Obstétrique, Centre Hospitalier Universitaire Sud Réunion, BP 350, CEDEX, 97448 Saint-Pierre, La Reunion, France 4 Department of Obstetrics & Gynaecology, Robinson Institute, Lyell McEwin Hospital, University of Adelaide, North Adelaide, SA 5005, Australia; [email protected] 5 Unit of Gynecological Surgery, Department of Obstetrics and Gynecology, Mater Dei Hospital, 70125 Bari, Italy; [email protected] Citation: Robillard, P.-Y.; Boukerrou, * Correspondence: [email protected]; Tel.: +262-2-62-35-91-49; Fax: +262-2-62-35-92-93 M.; Dekker, G.; Scioscia, M.; Bonsante, F.; Boumahni, B.; Iacobelli, S. Risk Abstract: Objectives: To develop a multivariate model for risk factors specific to early onset Factors for Early and Late Onset preeclampsia (EOP) and late onset preeclampsia (LOP) in our entire population (singleton and Preeclampsia in Reunion Island: twin pregnancies). -

Board-Review-Series-Obstetrics-Gynecology-Pearls.Pdf
ObstetricsandGynecology BOARDREVIEW Third Edition Stephen G. Somkuti, MD, PhD Associate Professor Department of Obstetrics and Gynecology and Reproductive Sciences Temple University School of Medicine School Philadelphia, Pennsylvania Director, The Toll Center for Reproductive Sciences Division of Reproductive Endocrinology Department of Obstetrics and Gynecology Abington Memorial Hospital Abington Reproductive Medicine Abington, Pennsylvania New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2008 by the McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-164298-6 The material in this eBook also appears in the print version of this title: 0-07-149703-X. All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. -

Multiple Pregnancy: Having More Than One Baby About This Information a Multiple Pregnancy Means You Are Having More Than One Baby at the Same Time
Information for you Published in September 2021 Multiple pregnancy: having more than one baby About this information A multiple pregnancy means you are having more than one baby at the same time. This is most commonly twins but may include triplets or, rarely, more. This information is for you if you are having a multiple pregnancy. It may also be helpful for your partner, family or friends. It focuses on twin and triplet pregnancies. If you are having more babies you can still use this information but you will have an individualised plan of care for your pregnancy depending on your circumstances. The information here aims to help you better understand your pregnancy and your options for treatment and care. Your healthcare team is there to support you in making decisions that are right for you. They can help by discussing your situation with you and answering your questions. 1 A glossary of medical terms is available on the RCOG website at: www.rcog.org.uk/en/patients/medical-terms. Key Points • Multiple pregnancy happens in about one in 60 pregnancies. • Most women with a multiple pregnancy will have a healthy pregnancy and will give birth to healthy babies, however complications are more common. • You will be offered extra antenatal checks and ultrasound scans to make sure that you are well and to monitor your babies closely. • If you have a multiple pregnancy you are more likely to give birth to your babies prematurely. • You will be advised to give birth in hospital. What is a multiple pregnancy? A multiple pregnancy is the term used when you are expecting two or more babies at the same time (twins, triplets or more). -
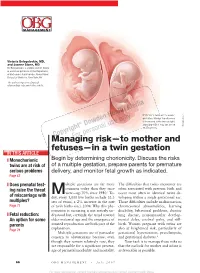
Managing Risk—To Mother and Fetuses—In a Twin Gestation
Victoria Belogolovkin, MD, and Joanne Stone, MD Dr. Belogolovkin is a fellow and Dr. Stone is associate professor in the Department of Maternal–Fetal Medicine, Mount Sinai School of Medicine, New York, NY The authors report no fi nancial relationships relevant to this article. Dichorionic twins at 11+ weeks' gestation. Nuchal translucency is increased in the twin at right, LaRocco ® signaling that it may be at risk Rich Dowden Health Media © of aneuploidy. Copyright ManagingFor personal risk—to use only mother and fetuses—in a twin gestation IN THIS ARTICLE ❙ Monochorionic Begin by determining chorionicity. Discuss the risks twins are at risk of of a multiple gestation, prepare parents for premature serious problems delivery, and monitor fetal growth as indicated. Page 67 ❙ Does prenatal test- ultiple gestations are far more The diffi culties that twins encounter are ing raise the threat common today than they once often associated with preterm birth and Mwere—up 70% since 1980.1 To- occur most often in identical twins de- of miscarriage with day, every 1,000 live births include 32.3 veloping within a single gestational sac. multiples? sets of twins, a 2% increase in the rate Those diffi culties include malformation, Page 77 of twin births since 2004. Why this phe- chromosomal abnormalities, learning nomenon is occurring is not entirely un- disability, behavioral problems, chronic ❙ Fetal reduction: derstood but, certainly, the trend toward lung disease, neuromuscular develop- An option for some older maternal age and the emergence of mental delay, cerebral palsy, and still- parents assisted reproduction are both part of the birth. -
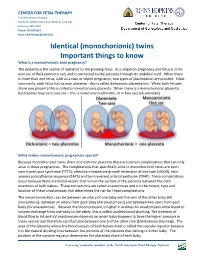
Monochorionic Twins Share One Common Placenta There Are Certain Complications That Can Only Arise in These Pregnancies
CENTER FOR FETAL THERAPY The Johns Hopkins Hospital 600 North Wolfe Street, Nelson Building, Suite 228 Baltimore, MD 21287 Phone: 410 502 6561 Email: [email protected] Identical (monochorionic) twins Important things to know What is a monochorionic twin pregnancy? The placenta is the source of nutrients to the growing fetus. In a singleton pregnancy one fetus is in his own sac of fluid (amniotic sac) and is connected to the placenta through its umbilical cord. When there is more than one fetus, such as a twin or triplet pregnancy, two types of placentation are possible. Most commonly, each fetus has its own placenta - this is called dichorionic placentation. When both fetuses share one placenta this is called a monochorionic placenta. When there is a monochorionic placenta both babies may be in one sac – this is called mono-amniotic, or in two sacs (di-amniotic). What makes monochorionic pregnancies special? Because monochorionic twins share one common placenta there are certain complications that can only arise in these pregnancies. The complications that specifically arise in monochorionic twins are twin- twin transfusion syndrome (TTTS), selective intrauterine growth restriction of one twin (sIUGR), twin anemia polycythemia sequence (TAPS) and twin reversed arterial perfusion (TRAP). These complications occur because there are blood vessels that run on the surface of the placenta between the cord insertions of both babies. These connections are called anastomoses and it is the nature, type and location of these anastomoses that determines the risk for these complications. The vessel connections can be between an artery of one baby and the vein of the other baby (AV anastomosis), between an artery from each baby (AA anastomosis) and between two veins from each baby (VV anastomosis). -

Monochorionic Twin Pregnancy
Monochorionic Twin Pregnancy Monochorionic Twin Pregnancy Congratulations!! You are expecting twins. About 1 in 80 pregnancies conceived are twin, what is special about your twins is that they appear to be monochorionic. What are monochorionic twins? About a 1/3 of twin pregnancies are monochorionic. These twins occur when one sperm fertilizes one egg which then splits into two babies (embryos). The babies share the same afterbirth (placenta) and outer sac (placental chorionic membrane) but usually have their own inner sac (amniotic membrane). What does this mean to me? Most women with twin pregnancies progress normally. But there is an increased risk of having problems than if this was a single pregnancy or if your babies did not share the same placenta (dichorionic). Occasionally it may not be possible to be sure whether your pregnancy is monochorionic or dichorionic. These pregnancies would be managed as a monochorionic pregnancy. You may experience the common discomforts of pregnancy earlier, such as: x Sickness and tiredness in early pregnancy. x Heartburn, backache, swollen ankles and haemorrhoids in later pregnancy. x Anaemia – blood tests will be taken at 12, 28 and 34 weeks to check for this. The main complications with twin pregnancies are: x Smaller babies and/or excessive fluid around the babies (polyhydramnios) x We will scan your babies regularly to assess their growth and the amount of fluid around them. x Premature labour – half of all twins will deliver early. x If you go into labour before 32-34 weeks you may need to be transferred to a hospital with neonatal intensive care facilities. -

Pregnancy Myths and Practical Tips Rebecca Caro, DO, Bassett Army Community Hospital, Fort Wainwright, Alaska Julia Fast, DO, Carl R
BONUS DIGITAL CONTENT Pregnancy Myths and Practical Tips Rebecca Caro, DO, Bassett Army Community Hospital, Fort Wainwright, Alaska Julia Fast, DO, Carl R. Darnall Army Medical Center Family Medicine Residency Program, Fort Hood, Texas For many patients, pregnancy is a highly anticipated and exciting phase of life, but it can also be anx- iety provoking. Family physicians can resolve some of this anxiety and promote maternal and fetal health by making specific recommendations at prenatal visits. A daily prenatal vitamin with at least 400 mcg of folic acid and 30 mg of elemental iron should be recommended to promote neurologic and musculoskeletal fetal development. Weight gain in pregnancy should be guided by preconcep- tion body mass index. People who are underweight should gain 28 to 40 lb, those who have a normal weight should gain 25 to 35 lb, and those who are overweight or obese should gain 15 to 25 lb or 11 to 20 lb, respectively. A well-balanced diet including omega-3 fatty acids should be encouraged. Unpas- teurized foods should be avoided during pregnancy because of the risk of listeriosis. Caffeine intake should be limited to 200 mg per day (about two small cups of coffee), and artificial sweeteners should be avoided. Pregnant patients should be encouraged to engage in regular cardiovascular activity for at least 150 minutes per week. Bed rest is not recommended. Sex can be continued throughout an uncomplicated pregnancy. Avoidance of alcohol and marijuana is recommended. The effects of hair dye or hair straightening products on fetal development or neonatal outcomes are unclear. -

Twin to Twin Transfusion Syndrome
1529 Review Article on Fetal Surgery Twin to twin transfusion syndrome Jena L. Miller^ The Johns Hopkins Center for Fetal Therapy, Department of Gynecology and Obstetrics, Johns Hopkins University, Baltimore, MD, USA Correspondence to: Jena L. Miller, MD. Assistant Professor, The Johns Hopkins Center for Fetal Therapy, Department of Gynecology and Obstetrics, 600 N Wolfe St, Nelson 228, Baltimore, MD 21287, USA. Email: [email protected]. Abstract: Twin to twin transfusion syndrome (TTTS) is a common complication that typically presents in the second trimester of pregnancy in 10–15% of monochorionic twins due to net transfer of volume and hormonal substances from one twin to the other across vascular anastomoses on the placenta. Without recognition and treatment, TTTS is the greatest contributor to fetal loss prior to viability in 90–100% of advanced cases. Ultrasound diagnosis of monochorionicity is most reliable in the first trimester and sets the monitoring strategy for this type of twins. The diagnosis of TTTS is made by ultrasound with the findings of polyhydramnios due to volume overload and polyuria in one twin and oligohydramnios due to oliguria of the co-twin. Assessment of bladder filling as well as arterial and venous Doppler patterns are required for staging disease severity. Assessment of fetal cardiac function also provides additional insight into the fetal cardiovascular impacts of the disease as well as help identify fetuses that may require postnatal follow up. Fetoscopic laser ablation of the communicating vascular anastomoses between the twins is the standard treatment for TTTS. It aims to cure the condition by interrupting the link between their circulations and making them independent of one another.