Torpor and Hypothermia: Reversed Hysteresis of Metabolic Rate and Body Temperature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cold-Hearted Bats: Uncoupling of Heart Rate and Metabolism During Torpor at Sub-Zero Temperatures Shannon E
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb170894. doi:10.1242/jeb.170894 RESEARCH ARTICLE Cold-hearted bats: uncoupling of heart rate and metabolism during torpor at sub-zero temperatures Shannon E. Currie1,2,*, Clare Stawski1,3 and Fritz Geiser1 ABSTRACT Depending on ambient conditions, animals will thermoconform Many hibernating animals thermoregulate during torpor and defend down to this Tb/Ta (Heller, 1979; Geiser, 2011). However, when Ta falls below Tcrit, animals can either rewarm entirely to a their body temperature (Tb) near 0°C by an increase in metabolic rate. normothermic Tb or remain torpid and increase metabolic heat Above a critical temperature (Tcrit), animals usually thermoconform. production to thermoregulate and defend minimum Tb. We investigated the physiological responses above and below Tcrit for a small tree-dwelling bat (Chalinolobus gouldii, ∼14 g) that is often Details regarding thermoregulation during torpor at sub-zero exposed to sub-zero temperatures during winter. Through temperatures are scant and restricted to medium-sized northern mammals such as ground squirrels (Geiser and Kenagy, 1988; Buck simultaneous measurement of heart rate (fH) and oxygen ̇ and Barnes, 2000; Richter et al., 2015). However, small hibernators, consumption (VO2), we show that the relationship between oxygen transport and cardiac function is substantially altered in such as temperate insectivorous bats, often use torpor year round thermoregulating torpid bats between 1 and −2°C, compared with (Eisentraut, 1956; Davis and Reite, 1967; Masing and Lutsar, 2007; Wojciechowski et al., 2007). Thermoregulation during torpor, while thermoconforming torpid bats at mild ambient temperatures (Ta 5–20° still conserving substantial amounts of energy, can be expensive C). -

WILDLIFE in WINTER Do You Know What Different Animals Do During the Winter?
WILDLIFE IN WINTER Do you know what different animals do during the winter? Animals have different strategies for surviving the winter. The most common strategies are hibernation, brumation, diapause, torpor, migration and adaptation. Hibernation Torpor Hibernation occurs when an animal enters a deep sleep Torpor is a state that some animals for the entire winter. Animals that hibernate eat extra food enter during the winter. Similar to during the fall to store up fat before winter begins. When it’s hibernation, the animal lowers its body time to hibernate, the animal drops its body temperature temperature and slows its breathing and by 20 °C or more and slows its heart rate and breathing in heart rate. However, animals that use torpor order to use less energy. during the winter may wake up occasionally or regularly to hunt, eat and defecate. Some animals Brumation are also able to go in and out of torpor regularly, like Brumation is a state of inactivity that cold-blooded creatures when it gets very cold at night. enter during winter. Think of this as hibernation for reptiles and amphibians. During extended cold periods, their bodies Migration produce high levels of sugar and slow or shutdown their Migration is the act of moving from one place to another. internal processes. Some animals can even freeze! Some creatures migrate to a warmer location when the weather gets too cold. They may travel alone or in large Diapause groups to areas where food is plentiful. Diapause occurs when insects pause their development to prepare for winter. Some insects stop all body processes and Adaptation sometimes freeze until the weather warms up in the spring, Adaptation to winter weather can take many different forms. -

Mechanisms of Animal Diapause: Recent Developments from Nematodes, Crustaceans, Insects, and fish
Am J Physiol Regul Integr Comp Physiol 310: R1193–R1211, 2016. First published April 6, 2016; doi:10.1152/ajpregu.00250.2015. Review Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish Steven C. Hand,1* David L. Denlinger,2* Jason E. Podrabsky,3* and Richard Roy4* 1Department of Biological Sciences, Louisiana State University, Baton Rouge, Louisiana; 2Departments of Entomology and Evolution, Ecology and Organismal Biology, Ohio State University, Columbus, Ohio; 3Department of Biology, Portland State University, Portland, Oregon; and 4Department of Biology, McGill University, Montréal, Québec, Canada Submitted 5 June 2015; accepted in final form 11 March 2016 Hand SC, Denlinger DL, Podrabsky JE, Roy R. Mechanisms of animal diapause: recent developments from nematodes, crustaceans, insects, and fish. Am J Physiol Regul Integr Comp Physiol 310: R1193–R1211, 2016. First published Downloaded from April 6, 2016; doi:10.1152/ajpregu.00250.2015.—Life cycle delays are beneficial for opportunistic species encountering suboptimal environments. Many animals display a programmed arrest of development (diapause) at some stage(s) of their development, and the diapause state may or may not be associated with some degree of metabolic depression. In this review, we will evaluate current advance- ments in our understanding of the mechanisms responsible for the remarkable phenotype, as well as environmental cues that signal entry and termination of the http://ajpregu.physiology.org/ state. The developmental stage at which diapause occurs dictates and constrains the mechanisms governing diapause. Considerable progress has been made in clarify- ing proximal mechanisms of metabolic arrest and the signaling pathways like insulin/Foxo that control gene expression patterns. -

Glacier National Park Winter Ecology Lessons
Winter Ecology Teacher’s Guide 2 Glacier National Park Table of Contents Page # Dear Teacher and Glacier Education Goals 3 Winter Ecology Background Information 4 - 13 What is Winter? 4 What Causes Winter 4 Snow 5 Adaptations 7 Insects 8 Animals in Winter 10 Humans in Winter 13 Activities and Lessons 14-48 What Causes Winter? 14 Dressing for Winter 16 What is Wild? 21 Surviving Winter in the Wild 23 Who Eats Who? 29 Hibernate, Migrate, or Resist? 34 Snug in the Snow 37 Snow Characteristics 39 How Much Water is in this Snow? 43 Tracks Along the Trail-Winter Signs 48 Supplementary Materials 54-76 Animal Card Clues & Drawings 54-57 Coloring book 58-66 Bird Checklist for Glacier National Park 67-70 Mammals Checklist for Glacier National Park 71-72 Vocabulary 73-75 Resources and References 76 Acknowledgements Thanks to all of the Glacier National Park Education Rangers and Interpretive Staff for help with this guide. It is a work in progress. Please direct corrections, comments, or additions to Laura Law, 888-5837, [email protected] . Cover artwork is by Robin Peterson and is avail- able as a color poster through the Glacier Natural History Association, 406-888-5756. Glacier National Park 3 Dear Teacher, A winter field trip to Glacier National Park offers unique opportunities for studying and ex- periencing plant and animal life in a different season. A fresh snowfall harbors many secrets. If you look closely you may see footprints and wing prints. Tracks, trails, and impressions in the snow are winter signs. They tell exciting stories that connect the past with the present. -

Winter Time in Nature
Classification Theme: To teach participants how to classify animals based on similarities they share. Background Learn the basics of the seven levels of calissifcation, Kingdom, Phylum, Class, Order Family, Genus and Species. (King Philip Come Out For Goodness Sake) Age K-5 The two mail Kingdoms are plants and animals. Four other Kingdoms include bacteria, Time 60 min archaebacterial, fungi and protozoa. Outline Phylum is separated into 30 phyla for animals. Phylum Chordata is characterized as Background the animals with backbones (Vertebrates) (chordate looks like a cord). Phylum Arthropods (Invertebrates) contains insects, spiders or any other animal with Activities: segmented bodies. They also have an exoskeleton on the outside of their bodies. Introduction Migration Class is separated into more sections within the Phylum. Example, Phylum Chordata Hibernation (animals with backbones) is then separated into birds, mammals and reptiles. Brumation Active animals Order is smaller groups within the different classes. For example, Lepidoptera is the Wrap up order of moths and butterflies. Lepidoptera is under the class insects. Carnivora is the Extra order of Mammalia. Information Family is often Speculated about as many different sources will disagree on the family of the animal. The family of dogs is Canidae (carnivorous mammals that include wolves, jackal, foxes, coyote and the domestic dog) Genus may only have one or two animals in it. If the animals are from the same genus, they are closely related. They may even look alike. When the genus is written it is capitalized and italicized. The genus of dogs and wolves is Canis. Species is when two animals can breed together successfully. -

Eastern Red Bat (Lasiurus Borealis) Response to Fire Stimulus
EASTERN RED BAT (LASIURUS BOREALIS) RESPONSE TO FIRE STIMULUS DURING TORPOR A Masters Thesis Presented to The Graduate College of Missouri State University In Partial Fulfillment Of the Requirements for the Degree Master of Science, Biology By Jason Thomas Layne May 2009 Copyright 2009 by Jason Thomas Layne ii EASTERN RED BAT (LASIURUS BOREALIS) RESPONSE TO FIRE STIMULUS DURING TORPOR Biology Missouri State University, May 2009 Master of Science Jason Thomas Layne ABSTRACT Current forest management practices during winter utilize prescribed fires to increase plant diversity and reduce competition for desired tree species. Concerns have arisen for eastern red bats (Lasiurus borealis) that utilize fallen leaf litter for roosting during cold (<10oC) temperatures. I studied responses of torpid leaf litter-roosting red bats (n = 18) to stimuli from fires in an experimental open area environment. Latencies of each behavior associated with awareness to the stimuli of fire (first response, arousal, and flight) were correlated to ambient weather parameters temperature, wind speed, and relative humidity at three different time periods. Latencies of all behaviors were significantly negatively correlated to temperatures, showing that higher temperatures corresponded to decreases in reaction times. Increased wind speeds just before the start and during a fire were correlated with latencies of first response and arousal behavior, and were also negatively correlated with latency of flight response throughout the time periods measured. Levels of carbon monoxide throughout a trial were smaller compared to laboratory smoke trials and data from an actual prescribed fire. I recommend conducting winter fires on days when temperatures are >10oC and starting the fire on a north-facing slope in order to give eastern red bats a chance to passively rewarm and react to an imposing fire. -

Seasonal Metabolic Depression, Substrate Utilisation and Changes In
The Journal of Experimental Biology 207, 307-318 307 Published by The Company of Biologists 2004 doi:10.1242/jeb.00756 Seasonal metabolic depression, substrate utilisation and changes in scaling patterns during the first year cycle of tegu lizards (Tupinambis merianae) Silvia Cristina R. de Souza1,*, José Eduardo de Carvalho1, Augusto S. Abe2, José Eduardo P. W. Bicudo1 and Marilene S. C. Bianconcini1 1Departamento de Fisiologia, Instituto de Biociências, Universidade de São Paulo, 05508-900 São Paulo, SP, Brazil and 2Departamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista, Caixa Postal 199, 13506-900 Rio Claro, SP, Brazil *Author for correspondence (e-mail: [email protected]) Accepted 16 October 2003 Summary The tegus increase in body mass after hatching until supplementary energy source in arousing animals. No early autumn, when the energy intake becomes gradually change is detectable in citrate synthase activity, but β- reduced. Resting rates of oxygen consumption in winter hydroxyacyl CoA dehydrogenase activities are strongly drop to 20% of the values in the active season affected by season, reaching a 3-fold and 5-fold increase . –1 –1 (VO·=0.0636·ml·g ·h ) and are nearly temperature in the liver tissue of winter and arousing animals, insensitive over the range of 17–25°C (Q10=1.55). During respectively, and becoming reduced by half in skeletal dormancy, plasma glucose levels are 60% lower than those muscle and heart of winter animals compared with late in active animals, while total protein, total lipids and β- fall or spring active individuals. From hatching to late hydroxybutyrate are elevated by 24%, 43% and 113%, autumn, the increase of the fat body mass relatively to respectively. -

Do Red Squirrels (Tamiasciurus Hudsonicus) Use Daily Torpor During Winter?1
19 (2): 127-132 (2012) Do red squirrels (Tamiasciurus hudsonicus) use daily torpor during winter?1 R. Mark BRIGHAM2, Department of Biology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada, e-mail: [email protected] Fritz GEISER, Centre for Behavioural and Physiological Ecology, Zoology, University of New England, Armidale NSW, 2351 Australia. Abstract: Given their relatively small body size, high thermoregulatory costs, and low metabolic rate, we tested the hypothesis that red squirrels (Tamiasciurus hudsonicus) would employ bouts of daily torpor to save energy during winter. We collected data on body temperature (Tb) using surgically implanted data loggers for squirrels in the Cypress Hills region of Saskatchewan, where extended periods of cold snowy weather make foraging difficult and should lead to high levels of energy expenditure. Based on over 8000 measurements from 4 animals over 3 winters, we found no evidence for torpor use. However, Tb was lowest in January and highest in September and May, and mean monthly Tb was correlated with mean monthly ambient temperature (Ta). Given that taxonomically related species can and do use torpor, it remains to be determined what makes heterothermy in this species costly to the extent that its use is precluded. Keywords: energetics, heterothermy, red squirrels, temperature dataloggers, torpor. Résumé : Étant donné leur relative petite taille corporelle, leurs coûts élevés de thermorégulation et leur faible taux métabolique, nous avons évalué l'hypothèse que les écureuils roux (Tamiasciurus hudsonicus) utiliseraient des périodes journalières de torpeur pour économiser de l'énergie en hiver. Nous avons récolté des données de température corporelle en utilisant des enregistreurs de données implantés par chirurgie chez des écureuils dans la région de Cypress Hills en Saskatchewan où des épisodes prolongés de temps froid et neigeux font que la quête alimentaire devient difficile et devraient ainsi entraîner des dépenses énergétiques élevées. -
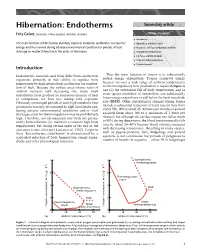
Hibernation: Endotherms Secondary Article
Hibernation: Endotherms Secondary article Fritz Geiser, University of New England, Armidale, Australia Article Contents . Introduction The main function of hibernation and daily torpor in mammals and birds is to conserve . Hibernation and Daily Torpor energy and thus survive during adverse environmental conditions or periods of food . Occurrence of Torpor in Mammals and Birds shortage no matter if they live in the arctic or the tropics. Preparation for Hibernation . Fat Stores and Dietary Lipids . Body Size and its Implications . Periodic Arousals Introduction Endothermic mammals and birds differ from ectothermic Thus the main function of torpor is to substantially organisms primarily in their ability to regulate body reduce energy expenditure. Torpor conserves energy temperature by high internal heat production via combus- because: (i) over a wide range of ambient temperatures tion of fuels. Because the surface area/volume ratio of no thermoregulatory heat production is required (Figure 1) animals increases with decreasing size, many small and (ii) the substantial fall of body temperature, and in endotherms must produce an enormous amount of heat some species inhibition of metabolism, can substantially to compensate for heat loss during cold exposure. lower energy expenditure to well below the basal metabolic Obviously, prolonged periods of such high metabolic heat rate (BMR). Other physiological changes during torpor production can only be sustained by high food intake and, include a substantial reduction of heart rate (in bats from during adverse environmental conditions and/or food about 500–900 to about 20–40 beats per minute; in ground shortages, costs for thermoregulation may be prohibitively squirrels from about 300 to a minimum of 3 beats per high. -
Advantage of Animal Models with Metabolic Flexibility for Space Research
Advantage of animal models with metabolic flexibility for space research beyond Low Earth Orbit Yuri V. Griko1*, Jon C. Rask2, Raycho Raychev3 1,2 NASA Ames Research Center, Moffett Field, CA 94035 3 Space Challenges Program, EnduroSat Inc. Sofia, Bulgaria *Corresponding author: Space Biosciences Division, NASA Ames Research Center, MS261-3, Moffett Field, CA 94035, USA. Tel: +1 650-604-0519; fax: +1 650-604-0046 Email: [email protected] (Y.V. Griko). 1 Abstract: As the world’s space agencies and commercial entities continue to expand beyond Low Earth Orbit (LEO), novel approaches to carry out biomedical experiments with animals are required to address the challenge of adaptation to space flight and new planetary environments. The extended time and distance of space travel along with reduced involvement of Earth-based mission support increases the cumulative impact of the risks encountered in space. To respond to these challenges, it becomes increasingly important to develop the capability to manage an organism’s self-regulatory control system, which would enable survival in extraterrestrial environments. To significantly reduce the risk to animals on future long duration space missions, we propose the use of metabolically flexible animal models as “pathfinders,” which are capable of tolerating the environmental extremes exhibited in spaceflight, including altered gravity, exposure to space radiation, chemically reactive planetary environments and temperature extremes. In this report we survey several of the pivotal metabolic flexibility studies and discuss the importance of utilizing animal models with metabolic flexibility with particular attention given to the ability to suppress the organism's metabolism in spaceflight experiments beyond LEO. -

Wildlife and Winter
Wildlife and Winter - modified from Snow Activity Program by John Pattimore The ecology of wildlife and winter is an intricate web of seasonal changes and adaptations by animals to these changes. Temperature and snow are the two ecological factors that present problems to wildlife in winter. The degree to which animals can cope with these factors depends on behavioral, physiological, and morphological adaptations. Many animals totally avoid cold winter temperatures by migrating, hibernating, or just sleeping. For some of these it is not the cold that directly causes this behavior. Birds have very adequate insulating feathers, but many birds migrate. A bear has thick fur, which is also a good insulator, but bears sleep in warm dens most of the winter. Still, many other animals remain completely active in winter. What dictates whether an animal will migrate or stay? What influences whether an animal will hibernate, sleep, or remain active? It takes more than good insulation to keep animals warm. They must have fuel to produce body heat. Energy, which most obtain through food, is the dictator. Cold temperatures kill or drive off much of the animals’ food, or snow (caused by cold temperatures) covers their food sources. Adaptations to Cold All animals must have a means of regulating body temperatures. Thermoregulation, as this is called, can be divided into three categories: poikilothermic, homeotherm, and heterotherms (as defined in Wildlife Document). Many homeotherms use torpor as a way to conserve energy when the temperature changes. Torpor is a state of decreased physiological activity in an animal, usually by a reduced body temperature and metabolic rate. -

Metabolic Flexibility: Hibernation, Torpor, and Estivation
Metabolic Flexibility: Hibernation, Torpor, and Estivation James F. Staples*1 ABSTRACT Many environmental conditions can constrain the ability of animals to obtain sufficient food en- ergy, or transform that food energy into useful chemical forms. To survive extended periods un- der such conditions animals must suppress metabolic rate to conserve energy, water, or oxygen. Amongst small endotherms, this metabolic suppression is accompanied by and, in some cases, fa- cilitated by a decrease in core body temperature—hibernation or daily torpor—though significant metabolic suppression can be achieved even with only modest cooling. Within some ectotherms, winter metabolic suppression exceeds the passive effects of cooling. During dry seasons, estivating ectotherms can reduce metabolism without changes in body temperature, conserving energy re- serves, and reducing gas exchange and its inevitable loss of water vapor. This overview explores the similarities and differences of metabolic suppression among these states within adult animals (excluding developmental diapause), and integrates levels of organization from the whole animal to the genome, where possible. Several similarities among these states are highlighted, including patterns and regulation of metabolic balance, fuel use, and mitochondrial metabolism. Differences among models are also apparent, particularly in whether the metabolic suppression is intrinsic to the tissue or depends on the whole-animal response. While in these hypometabolic states, tis- sues from many animals are tolerant of hypoxia/anoxia, ischemia/reperfusion, and disuse. These natural models may, therefore, serve as valuable and instructive models for biomedical research. © 2016 American Physiological Society. Compr Physiol 6:737-771, 2016. Introduction the gas exchange required to support aerobic metabolism is associated with some inevitable loss of water vapor from gas Metabolic energy is required for animal development, exchange surfaces.