In the Court of Chancery of the State of Delaware Jeff
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

XCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) (X) ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 ( ) TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 0-19034 REGENERON PHARMACEUTICALS, INC. (Exact name of registrant as specified in its charter) New York 13-3444607 (State or other jurisdiction of (I.R.S. Employer Identification No) incorporation or organization) 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707 (Address of principal executive offices) (Zip code) (914) 347-7000 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: None (Title of Class) Securities registered pursuant to Section 12(g) of the Act: Common Stock - par value $.001 per share (Title of Class) Preferred Share Purchase Rights expiring October 18, 2006 (Title of Class) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes /X/ No Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (ss. 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. -

Virginia: in the Circuit Court of Pittsylvania County
VIRGINIA: IN THE CIRCUIT COURT OF PITTSYLVANIA COUNTY PITTSYLVANIA COUNTY, Plaintiff, v. PURDUE PHARMA, L.P.; PURDUE PHARMA, INC.; THE PURDUE FREDERICK COMPANY, INC.; RHODES PHARMACEUTICALS, L.P.; ABBOTT LABORATORIES; ABBOTT LABORATORIES, INC.; MALLINCKRODT PLC; MALLINCKRODT LLC; ENDO HEALTH SOLUTIONS, INC; ENDO PHARMACEUTICALS, INC.; PAR Case No. CL18 - __________ PHARMACEUTICAL COMPANIES, INC.; PAR PHARMACEUTICAL, INC.; TEVA Jury Trial Demanded PHARMACEUTICALS USA, INC.; CEPHALON, INC.; BARR LABORATORIES, INC.; JANSSEN PHARMACEUTICALS, INC.; ORTHO-MCNEIL-JANSSEN PHARMACEUTICALS, INC.; JANSSEN PHARMACEUTICA, INC.; WATSON LABORATORIES, INC.; ALLERGAN PLC; ACTAVIS PHARMA, INC.; ACTAVIS, LLC; INSYS THERAPEUTICS, INC.; KVK-TECH, INC.; AMNEAL PHARMACEUTICALS LLC; IMPAX LABORATORIES, LLC; AMNEAL PHARMACEUTICALS, INC.; AMNEAL PHARMACEUTICALS OF NEW YORK, LLC; MYLAN PHARMACEUTICALS, INC.; MCKESSON CORPORATION; MCKESSON MEDICAL-SURGICAL INC.; CARDINAL HEALTH, INC.; AMERISOURCEBERGEN DRUG CORPORATION; HENRY SCHEIN, INC.; GENERAL INJECTABLES & VACCINES, INC.; INSOURCE, INC.; CVS HEALTH CORPORATION; CVS PHARMACY, INC.; CVS TN DISTRIBUTION, L.L.C.; WALGREENS BOOTS ALLIANCE, INC.; WALGREEN CO.; EXPRESS SCRIPTS HOLDING COMPANY; EXPRESS SCRIPTS, INC; CAREMARK RX, L.L.C.; CAREMARKPCS HEALTH, L.L.C.; CAREMARK, L.L.C.; UNITEDHEALTH GROUP INCORPORATED; OPTUM, INC.; OPTUMRX, INC.; and DOES 1-100, Defendants. PLAINTIFF’S ORIGINAL COMPLAINT Plaintiff, Pittsylvania County, Virginia, by and through the undersigned attorneys, (hereinafter “Plaintiff,” “Pittsylvania -

Surescripts, Llc As Amicus Curiae in Support of Petitioners in No
Nos. 19-508 and 19-825 In the Supreme Court of the United States ———————————— AMG CAPITAL MANAGEMENT, LLC, ET AL., Petitioners, v. FEDERAL TRADE COMMISSION, Respondent. ———————————— FEDERAL TRADE COMMISSION, Petitioner, v. CREDIT BUREAU CENTER, LLC, ET AL., Respondents. ———————————— ON WRITS OF CERTIORARI TO THE UNITED STATES COURTS OF APPEALS FOR THE SEVENTH AND NINTH CIRCUITS ———————————— BRIEF OF SURESCRIPTS, LLC AS AMICUS CURIAE IN SUPPORT OF PETITIONERS IN NO. 19-508 AND RESPONDENTS IN NO. 19-825 ———————————— ALFRED C. PFEIFFER, JR. ROMAN MARTINEZ LATHAM & WATKINS LLP Counsel of Record 505 Montgomery Street AMANDA P. REEVES Suite 2000 ALLYSON M. MALTAS San Francisco, CA 94111 DOUGLAS C. TIFFT BLAKE E. STAFFORD JAMES A. TOMBERLIN* LATHAM & WATKINS LLP 555 Eleventh Street, NW Suite 1000 Washington, DC 20004 (202) 637-2200 [email protected] Counsel for Amicus Curiae Surescripts, LLC TABLE OF CONTENTS Page TABLE OF AUTHORITIES ...................................... ii INTEREST OF AMICUS CURIAE ............................1 SUMMARY OF ARGUMENT .....................................3 ARGUMENT ...............................................................5 I. The FTC Has Increasingly Wielded Section 13(b) To Obtain Monetary Relief In Antitrust Cases ................................................5 II. The FTC’s Antitrust Authority Confirms That Section 13(b) Does Not Authorize Monetary Relief ..................................................22 CONCLUSION ..........................................................32 ii TABLE OF AUTHORITIES Page(s) CASES Apple Inc. v. Pepper, 139 S. Ct. 1514 (2019) .......................................... 13 Armstrong v. Exceptional Child Center, Inc., 575 U.S. 320 (2015) .............................................. 23 Bell Atlantic Corp. v. Twombly, 550 U.S. 544 (2007) .............................................. 26 In re Cardinal Health, Inc., No. 101-0006, 2015 WL 1849040 (F.T.C. Apr. 17, 2015) ........................ 19, 20, 28, 30 Credit Suisse Securities (USA) LLC v. Billing, 551 U.S. -

Complaint, “Chronic Pain” Means Non-Cancer Pain Lasting Three Months Or Longer
TABLE OF CONTENTS Page I. INTRODUCTION ...............................................................................................................1 II. JURISDICTION AND VENUE ..........................................................................................8 III. PARTIES .............................................................................................................................8 A. Plaintiff ....................................................................................................................8 B. Defendants ...............................................................................................................9 IV. FACTUAL ALLEGATIONS ............................................................................................14 A. Defendants Used Multiple Avenues To Disseminate Their False And Deceptive Statements About Opioids. ...................................................................14 1. Defendants spread and continue to spread their false and deceptive statements through direct marketing of their branded opioids. .......................................................................................................15 2. Defendants used a diverse group of seemingly independent third parties to spread false and deceptive statements about the risks and benefits of opioids.................................................................17 a. Key Opinion Leaders (“KOLs”) ....................................................19 (1) Russell Portenoy ................................................................20 -

Non-Merger Civil Enforcement: an Overview of Recent DOJ and FTC Federal Court Litigation
Antitrust , Vol. 32, No. 1, Fall 201 7. © 2017 by the American Bar Association. Reproduced with permission. All rights reserved. This information or any portion thereof may not be copied or disseminated in any form or by any means or stored in an electronic database or retrieval system without the express written consent of the American Bar Association. Non-Merger Civil Enforcement: An Overview of Recent DOJ and FTC Federal Court Litigation BY SONIA KUESTER PFAFFENROTH ECENT YEARS HAVE SEEN THE As a result, there are now a significant number of career attor - Department of Justice and the Federal Trade neys and economists with recent federal trial court experience, Commission appearing with regularity in fed - which they will bring to future cases at the investigative phase eral district court, with the agencies demon - with an eye towards potential litigation. strating a willingness to litigate in both the Rmerger and non-merger context and with a number of high- DOJ Litigation profile trials now in the rearview mirror. Because the DOJ has no administrative adjudicative process, While the majority of civil conduct enforcement actions its civil enforcement cases, whether they are settlements or continue to be filed concurrently with settlements—which contested litigation, are filed directly in federal district court. provide significant insight into the government’s theories— In recent years, the DOJ has litigated a number of cases alleg - both agencies have seen an uptick in the number of contest - ing Section 1 violations and one case alleging a Section 2 vio - ed cases filed in federal district court since the beginning of lation. -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

November 26, 2018 Dr. Sol Barer Chairman of the Board Teva
November 26, 2018 Dr. Sol Barer Chairman of the Board Teva Pharmaceutical Industries Limited Basel Street, P.O. Box 3190, Petach Tikva 4951033, Israel Dear Dr. Barer, We write to you as members of the Investors for Opioid Accountability (IOA) which represents a diverse global coalition of public, faith-based, labor, and sustainable funds, as well as asset managers with $2.2 trillion in assets under management.1 The IOA came together in 2017 out of concern for the potential risks that opioids present for the companies in which we invest. As global investors, we are writing to request a meeting with you to discuss potential financial, legal and reputational risks Teva Pharmaceutical Industries Limited (“Teva”) is facing related to the manufacturing and sale of opioids, and to ask that the Board consider adopting governance reforms designed to mitigate those risks. Opioid abuse is undeniably a public health crisis across North America and now spreading outside the U.S. as well. The U.S. Centers for Disease Control and Prevention (“CDC”) report that in 2016 alone, opioid abuse caused 42,249 deaths in the United States, or 115 people per day. In Canada, there were approximately 4,000 opioid-related deaths in 2017, a 34% increase from the prior year and a rate of 10.9 deaths per 100,000 people. In the UK and Wales, people dying from opioids related deaths in 2016 reached a record high of 3,700. In the U.S., the economic and social effects of the opioid crisis have been profound. Economist Alan Krueger has estimated that nearly 50% of prime age non-labor force men take prescription medication on a daily basis, with almost two-thirds of these being prescription pain medication. -
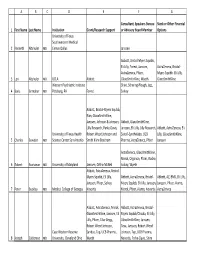
1 2 3 4 5 6 7 8 ABCDEFG First Name Last Name
ABC D E F G Consultant, Speakers Bureau Stock or Other Financial 1 First Name Last Name Institution Grant/Research Support or Advisory Board Member Options University of Texas Southwestern Medical 2 Kenneth Altshuler MD Center Dallas Janssen Abbott, Bristol‐Myers Squibb, Eli Lilly, Forest, Janssen, AstraZeneca, Bristol‐ AstraZeneca, Pfizer, Myers Squibb. Eli Lilly, 3 Lori Altshuler MD UCLA Abbott GlaxoSmithKline, Wyeth GlaxoSmithKline Western Psychiatric Institute Shire, Schering‐Plough, Jazz, 4 Boris Birmaher MD Pittsburg, PA Forest Solvay Abbott, Bristol‐Myers Squibb, Elan, GlaxoSmithKline, Janssen, Johnson & Johnson, Abbott, GlaxoSmithKline, Lilly Research, Parke‐Davis, Janssen, Eli Lilly, Lilly Research, Abbott, AstraZeneca, Eli University of Texas Health Robert Wood Johnson and Sanofi‐Synthelabo, UCB Lilly, GlaxoSmithKline, 5 Charles Bowden MD Science Center San Antonio Smith Kline Beecham Pharma, AstraZeneca, Pfizer Janssen AstraZeneca, GlaxoSmithKline, Merck, Organon, Pfizer, Roche, 6 Robert Buchanan MD University of Maryland Janssen, Ortho‐McNeil Solvay, Wyeth Abbott, AstraZeneca, Bristol‐ Myers Squibb, Eli Lilly, Abbott, AstraZeneca, Bristol‐ Abbott, AZ, BMS, Eli Lilly, Janssen, Pfizer, Solvay, Myers Squibb, Eli Lilly, Janssen, Janssen, Pfizer, Alamo, 7 Peter Buckley MD Medical College of Georgia Novartis Merck, Pfizer, Alamo, Novartis AstraZeneca Abbott, AstraZeneca, Merck, Abbott, AstraZeneca, Bristol‐ GlaxoSmithKline, Janssen, Eli Myers Squibb/Otsuka, Eli Lilly, Lilly, Pfizer, Ciba‐Geigy, GlaxoSmithKline, Janssen, Robert Wood -

Oral Presentation Disclosures
Oral Presentation Disclosures Adler, Lenard – Alcobra Pharma, APSARD/Pond Foundation, Major League Baseball, Major League Baseball Players Association, National Football League, New York University School of Medicine, Novartis Bioventures, Shire Pharmaceuticals, Sunovion, SUNY Upstate, Theravance, US Department of Veterans Affairs Cooperative Studies Program Anton, Raymond – Abbvie, Alkermes, Eli Lilly, Ethypharm, Lundbeck, Pfizer, Sunpharma Baker, Ross – Otsuka Pharmaceutical Development & Commercialization, Inc. Baldwin, David – Lundbeck Beaver, Jessica – Targacept, Inc. Bencherif, Merouane – Targacept, Inc. Bertolino, Alessandro – F. Hoffmann-La Roche, Ltd. Bradshaw, Mark – Euthymics Bioscience, Neurovance, Inc. Burdick, Katherine – Dainippon Sumitomo Pharma Bymaster, Frank – Euthymics Bioscience, Neurovance, Inc. Calabrese, Joseph – Sunovion, Teva (Cephalon) Cantillon, Marc – Forest, Kyowa, Lilly, Merck, Pfizer, Reviva Caroff, Stanley – Sunovion Chen, Yinzhong – Takeda Development Center Americas, Inc. Chengappa, Roy – Pfizer, Inc. Childress, Ann – Abbott Laboratories, Bristol Myer Squibb, GlaxoSmithKline, Ironshore, Janssen (Ortho-McNeil), Johnson & Johnson PRD, Lilly, Neos Therapeutics, Neurovance Inc., NextWave, Novartis, Noven, Otsuka, Pfizer, Rhodes, Sepracor, Shionogi, Shire, Somerset, Sunovion, Theravance Christine, Mazzucco – Janssen Cohen, Lee – Astra-Zeneca Pharmaceuticals, Bristol-Myers Squibb, Cephalon, Inc., GlaxoSmithKline, National Institute of Mental Health, National Institute on Aging, Noven Pharmaceuticals, Ortho-McNeil -

Supreme Court of the State of New York
Margaret H. Olson (Bar No. 6296) SUMMIT COUNTY ATTORNEY [email protected] 60 North Main Street Coalville, Utah 84017 (435)336-3206 Colin P. King (Bar No. 1815) Michael A. Worel (Bar No. 12741) [email protected] [email protected] DEWSNUP KING OLSEN WOREL HAVAS MORTENSEN 36 South State Street, Suite 2400 Salt Lake City, Utah 84111-0024 Telephone: (801) 533-0400 Donald J. Winder (Bar No. 3519) Edgar R. Cataxinos (Bar No. 7162) James E. Magleby (Bar No. 7247) Matthew B. McCune (Bar No. 15895) [email protected] [email protected] MAGLEBY CATAXINOS & GREENWOOD 170 South Main Street, Ste 1100 Salt Lake City, Utah 84101 Telephone: (801)359-9000 Attorneys for Plaintiff Summit County IN THE THIRD DISTRICT COURT OF THE DISTRICT OF THE STATE OF UTAH, IN AND FOR THE COUNTY OF SUMMIT, SILVER SUMMIT DIVISION SUMMIT COUNTY UTAH ) Coalville, UT ) Plaintiff, ) v. ) ) CASE NO.: 180500119 PURDUE PHARMA L.P. ) c/o The Prentice Hall Corporation ) 2711 Centerville Road ) Wilmington, DE 19808 ) ) and ) COMPLAINT FOR DAMAGES ) PURDUE PHARMA INC. ) (JURY DEMAND) c/o The Prentice Hall Corporation ) 2711 Centerville Road ) TIER 3 Wilmington, DE 19808 ) ) Judge: Kent Holmberg and ) ) THE PURDUE FREDERICK COMPANY, INC. ) c/o The Prentice Hall Corporation ) 2711 Centerville Road ) Wilmington, DE 19808 ) ) and ) ) TEVA PHARMACEUTICALS USA, INC. ) c/o Corporate Creations Network Inc. ) 3411 Silverside Road ) Wilmington, DE 19180 ) ) and ) ) CEPHALON, INC. ) c/o Corporate Creations Network Inc. ) 3411 Silverside Road ) Wilmington, DE 19180 ) ) and ) ) JOHNSON & JOHNSON ) One Johnson & Johnson Plaza ) New Brunswick, NJ 08933 ) ) and ) ) JANSSEN PHARMACEUTICALS, INC. ) 116 Pine Street, Suite 320 ) Harrisburg, PA 17101 ) ) and ) ) ORTHO-MCNEIL-JANSSEN ) PHARMACEUTICALS, INC. -

In the United States District Court for the District Of
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE PFIZER INC;, et al., ) ) Plaintiffs, ) ) v. ) Civ. No. 15-960-SLR ) MYLAN INC., et al., ) ) Defendants. ) ) Jack B. Blumenfeld, Esquire and Maryellen Noreika, Esquire of Morris Nichols, Arsht & Tunnell LLP, Wilmington, Delaware. Counsel for Plaintiffs. David E. Moore, Esquire, Bindu A. Palapura, Esquire, and Stephanie E. O'Byrne, Esquire of Potter Anderson & Corroon LLP, Wilmington, Delaware. Counsel for Defendants. Of Counsel: Robert L. Florence, Esquire, Karen L. Carroll, Esquire, and Micheal L. Binns, Esquire, Melanie Black Dubis, Esquire of Parker, Poe, Adams & Bernstein LLP. MEMORANDUM OPINION Dated: August \} , 2016 Wilmington, Delaware ROBINSON~ I. INTRODUCTION On October 22, 2015, Pfizer Inc., Wyeth LLC, Pfizer Pharmaceuticals LLC, PF PRISM C.V., ~nd Pfizer Manufacturing Holdings LLC, (collectively "plaintiffs") filed a complaint alleging infringement of three patents related to its injectable antibiotic product TYGACIL ® ("Tygacil") against defendants Mylan Inc., Mylan N.V., Mylan Laboratories Ltd. ("MLL"), and Mylan Pharmaceuticals ("MPI") (collectively "defendants"). (D.I. 1) Presently before the court is defendants' motion to dismiss for lack of personal jurisdiction and improper venue. (D.I. 6) Defendants Mylan Inc., Mylan N.V., and MPI have also filed a motion dismiss for failure to state a claim. (Id.) The court has jurisdiction over this matter pursuant to 28 U.S.C. §§ 1331 and 1338(a). II. BACKGROUND A. The parties Plaintiff Pfizer Inc. is a corporation organized and existing under the laws of the State of Delaware with a principal place of business in New York, New York. (D.I. 1 at~ 2) Plaintiff Wyeth LLC is a Delaware limited liability company with a principal place of business in New York, New York. -

Rxoutlook® 4Th Quarter 2020
® RxOutlook 4th Quarter 2020 optum.com/optumrx a RxOutlook 4th Quarter 2020 While COVID-19 vaccines draw most attention, multiple “firsts” are expected from the pipeline in 1Q:2021 Great attention is being given to pipeline drugs that are being rapidly developed for the treatment or prevention of SARS- CoV-19 (COVID-19) infection, particularly two vaccines that are likely to receive emergency use authorization (EUA) from the Food and Drug Administration (FDA) in the near future. Earlier this year, FDA issued a Guidance for Industry that indicated the FDA expected any vaccine for COVID-19 to have at least 50% efficacy in preventing COVID-19. In November, two manufacturers, Pfizer and Moderna, released top-line results from interim analyses of their investigational COVID-19 vaccines. Pfizer stated their vaccine, BNT162b2 had demonstrated > 90% efficacy. Several days later, Moderna stated their vaccine, mRNA-1273, had demonstrated 94% efficacy. Many unknowns still exist, such as the durability of response, vaccine performance in vulnerable sub-populations, safety, and tolerability in the short and long term. Considering the first U.S. case of COVID-19 was detected less than 12 months ago, the fact that two vaccines have far exceeded the FDA’s guidance and are poised to earn EUA clearance, is remarkable. If the final data indicates a positive risk vs. benefit profile and supports final FDA clearance, there may be lessons from this accelerated development timeline that could be applied to the larger drug development pipeline in the future. Meanwhile, drug development in other areas continues. In this edition of RxOutlook, we highlight 12 key pipeline drugs with potential to launch by the end of the first quarter of 2021.