Thesis Submitted DOCTOR of PHILOSOPHY the Faculty Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accused Persons Arrested in Thrissur City District from 12.06.2016 to 18.06.2016
Accused Persons arrested in Thrissur City district from 12.06.2016 to 18.06.2016 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 KOLAPPULLY HOUSE, 2078/16 U/S TOWN EAST 12.06.2016 M K AJAYAN, SI BAILED BY 1 RAGESH K R RAJAN 29 MALE MULAYAM P O, DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR at 00.15 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) AMBATT HOUSE, 2079/16 U/S TOWN EAST 12.06.2016 M K AJAYAN, SI BAILED BY 2 VARGHESE A T THOMAS 46 MALE MULAYAM P O , DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR at 00.22 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) MELAYIL HOUSE, 2080/16 U/S TOWN EAST RAMACHAND 12.06.2016 M K AJAYAN, SI BAILED BY 3 RAMAN 47 MALE MULAYAM P O , DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR RAN at 00.30 OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) MULLOOKKARAN 2081/16 U/S T OWN EAST 12.06.2016 M.K. AJAYAN, BAILED BY 4 SHIJI RAPPAI 39 MALE HOUSE, MULAYAM DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR AT 00.29 SI OF POLICE POLICE VALAKKAVU ABKARAI ACT CITY) PALUKKASSERY 2082/16 U/S TOWN EAST CHANDRASEKH 12.06.2016 M K AJAYAN, SI BAILED BY 5 RAJKUMAR 48 MALE HOUSE, MULAYAM P DIVANJIMOOLA 15(C) R/W 63 PS (THRISSUR ARAN at 00.50 OF POLICE POLICE O , VALAKKAVU ABKARAI ACT CITY) THACHATTIL HOUSE,NEAR 2084/16 U/S TOWN EAST V.K. -

Accused Persons Arrested in Thrissur City District from 13.09.2015 to 19.09.2015
Accused Persons arrested in Thrissur city district from 13.09.2015 to 19.09.2015 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 MECHERIPADI (H), NEDUPUZHA V B VALSAN, 13-09-15 at 1370/15 U/S 118 BAILED BY 1 EDWIN DAVIS 23 MALE MARATHAKKARA P O. VADOOKKARA PS (THRISSUR ADDL.SI OF 09.55 (a) KP Act POLICE PH 9947699359 CITY) POLICE KARIPPIYATH (H), NEDUPUZHA, NEDUPUZHA U RAJAN, HERBERT NAGAR, 13-09-15 at 1371/15 U/S 118 BAILED BY 2 JAYMON KUTTAPPAN 49 MALE HERBERT PS (THRISSUR ADDL.SI OF NEDUPUZHA. PH 10.10 (a) KP Act POLICE NAGAR CITY) POLICE 9656253772 UPPIKKERIL (H), 1372/15 U/S 279 NEDUPUZHA U RAJAN, GANDHI NAGAR, 13-09-15 at BAILED BY 3 SUBASH SUKUMARAN 42 MALE VADOOKKARA IPC AND 185 MV PS (THRISSUR ADDL.SI OF VADOOKKARA. PH 10.50 POLICE ACT CITY) POLICE 9539165566 VADAKKUMPARAMBIL NEDUPUZHA U RAJAN, (H0, VATTAPPINNI, VATTAPPINNI, 13-09-15 at 1374/15 U/S 15 BAILED BY 4 SHINEESH SREENIVASAN 28 MALE PS (THRISSUR ADDL.SI OF NEDUPUZHA. PH A K G NAGAR 16.20 OF KG ACT POLICE CITY) POLICE 8111850156 KARAYIL (H), NEDUPUZHA U RAJAN, VATTAPPINNI, VATTAPPINNI, 13-09-15 at 1374/15 U/S 15 BAILED BY 5 PRATHEESH SUKUMARAN 33 MALE PS (THRISSUR ADDL.SI OF NEDUPUZHA. -

List of Teachers Posted from the Following Schools to Various Examination Centers As Assistant Superintendents for Higher Secondary Exam March 2015
LIST OF TEACHERS POSTED FROM THE FOLLOWING SCHOOLS TO VARIOUS EXAMINATION CENTERS AS ASSISTANT SUPERINTENDENTS FOR HIGHER SECONDARY EXAM MARCH 2015 08001 - GOVT SMT HSS,CHELAKKARA,THRISSUR 1 DILEEP KUMAR P V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04884231495, 9495222963 2 SWAPNA P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9846374117 3 SHAHINA.K 08035-GOVT. RSR VHSS, VELUR, THRISSUR 04885241085, 9447751409 4 SEENA M 08041-GOVT HSS,PAZHAYANNOOR,THRISSUR 04884254389, 9447674312 5 SEENA P.R 08046-AKM HSS,POOCHATTY,THRISSUR 04872356188, 9947088692 6 BINDHU C 08062-ST ANTONY S HSS,PUDUKAD,THRISSUR 04842331819, 9961991555 7 SINDHU K 08137-GOVT. MODEL HSS FOR GIRLS, THRISSUR TOWN, , 9037873800 THRISSUR 8 SREEDEVI.S 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9020409594 9 RADHIKA.R 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742552608, 9847122431 10 VINOD P 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR , 9446146634 11 LATHIKADEVI L A 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04742482838, 9048923857 12 REJEESH KUMAR.V 08015-GOVT HSS,CHERUTHURUTHY,THRISSUR 04762831245, 9447986101 08002 - GOVT HSS,CHERPU,THRISSUR 1 PREETHY M K 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR 04802820505, 9496288495 2 RADHIKA C S 08003-GOVT MODEL GHSS, IRINJALAKKUDA, THRISSUR , 9495853650 3 THRESSIA A.O 08005-GOVT HSS,KODAKARA,THRISSUR 04802726280, 9048784499 4 SMITHA M.K 08046-AKM HSS,POOCHATTY,THRISSUR 04872317979, 8547619054 5 RADHA M.R 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872342425, 9497180518 6 JANITHA K 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872448686, 9744670871 1 7 SREELEKHA.E.S 08050-ST ANTONY S HSS,AMMADAM,THRISSUR 04872343515, 9446541276 8 APINDAS T T 08095-ST. PAULS CONVENT EHSS KURIACHIRA, THRISSUR, 04872342644, 9446627146 680006 9 M.JAMILA BEEVI 08107-SN GHSS, KANIMANGALAM, THRISSUR, 680027 , 9388553667 10 MANJULA V R 08118-TECHNICAL HSS, VARADIAM, THRISSUR, 680547 04872216227, 9446417919 11 BETSY C V 08138-GOVT. -

State District Branch Address Centre Ifsc Contact1 Contact2 Contact3 Micr Code Andhra Pradesh East Godavari Rajamundry Pb No
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE M RAGHAVA RAO E- MAIL : PAUL RAJAMUN KAKKASSERY PB NO 23, FIRST DRY@CSB E-MAIL : FLOOR, STADIUM .CO.IN, RAJAMUNDRY ROAD, TELEPHO @CSB.CO.IN, ANDHRA EAST RAJAMUNDRY, EAST RAJAHMUN NE : 0883 TELEPHONE : PRADESH GODAVARI RAJAMUNDRY GODAVARY - 533101 DRY CSBK0000221 2421284 0883 2433516 JOB MATHEW, SENIOR MANAGER, VENKATAMATTUPAL 0863- LI MANSION,DOOR 225819, NO:6-19-79,5&6TH 222960(DI LANE,MAIN R) , CHANDRAMOH 0863- ANDHRA RD,ARUNDELPET,52 GUNTUR@ ANAN , ASST. 2225819, PRADESH GUNTUR GUNTUR 2002 GUNTUR CSBK0000207 CSB.CO.IN MANAGER 2222960 D/NO 5-9-241-244, Branch FIRST FLOOR, OPP. Manager GRAMMER SCHOOL, 040- ABID ROAD, 23203112 e- HYDERABAD - mail: ANDHRA 500001, ANDHRA HYDERABA hyderabad PRADESH HYDERABAD HYDERABAD PRADESH D CSBK0000201 @csb.co.in EMAIL- SECUNDE 1ST RABAD@C FLOOR,DIAMOND SECUNDER SB.CO.IN TOWERS, S D ROAD, ABAD PHONE NO ANDHRA SECUNDERABA DECUNDERABAD- CANTONME 27817576,2 PRADESH HYDERABAD D 500003 NT CSBK0000276 7849783 THOMAS THARAYIL, USHA ESTATES, E-MAIL : DOOR NO 27.13.28, VIJAYAWA NAGABHUSAN GOPALAREDDY DA@CSB. E-MAIL : ROAD, CO.IN, VIJAYAWADA@ GOVERNPOST, TELEPHO CSB.CO.IN, ANDHRA VIJAYAWADA - VIJAYAWAD NE : 0866 TELEPHONE : PRADESH KRISHNA VIJAYAWADA 520002 A CSBK0000206 2577578 0866 2571375 MANAGER, E-MAIL: NELLORE ASST.MANAGE @CSB.CO. R, E-MAIL: PB NO 3, IN, NELLORE@CS SUBEDARPET ROAD, TELEPHO B.CO.IN, ANDHRA NELLORE - 524001, NE:0861 TELEPHONE: PRADESH NELLORE NELLORE ANDHRA PRADESH NELLORE CSBK0000210 2324636 0861 2324636 BR.MANAG ER : PHONE :040- ASST.MANAGE 23162666 R : PHONE :040- 5-222 VIVEKANANDA EMAIL 23162666 NAGAR COLONY :KUKATPA EMAIL ANDHRA KUKATPALLY KUKATPALL LLY@CSB. -

Conservation and Wise Use of Vembanad-Kol an Integrated Management Planning Framework
Conservation and Wise use of Vembanad-Kol An Integrated Management Planning Framework Wetlands International - South Asia Wetlands International – South Asia Mangroves for the Future WISA is the South Asia Programme of MFF is a unique partner- led initiative to Wetlands International, a global organization promote investment in coastal ecosystem dedicated to conservation and wise use of conservation for sustainable wetlands. Its mission is to sustain and development. It provides a collaborative restore wetlands, their resources and platform among the many different biodiversity. WISA provides scientific and agencies, sectors and countries who are technical support to national governments, addressing challenges to coastal wetland authorities, non government ecosystem and livelihood issues, to work organizations, and the private sector for towards a common goal. wetland management planning and implementation in South Asia region. It is MFF is led by IUCN and UNDP, with registered as a non government organization institutional partners : CARE, FAO, UNEP, under Societies Registration Act and steered and Wetlands International and financial by eminent conservation planners and support from Norad and SIDA wetland experts. Wetlands International-South Asia A-25, (Second Floor), Defence Colony New Delhi – 110024, India Telefax: +91-11-24338906 Email: [email protected] URL: http://south-asia.wetlands.org Conservation and Wise Use of Vembanad-Kol An Integrated Management Planning Framework Wetlands International – South Asia December 2013 Wetlands International - South Asia Project Team Acknowledgements Dr. Ritesh Kumar (Project Leader) Wetlands International – South Asia thanks the following individuals and organizations for support extended to management planning of Prof. E.J.James (Project Advisor) Vembanad-Kol wetlands Dr. -

Ss of the Sector Product (S) Enterprise # 1 Haritham Arts&Crafts Traditional Handicraft Thrikaipetta (Po), Nellimalam, Wayanad T-9495240567
NAME & ADDRESS OF THE SECTOR PRODUCT (S) ENTERPRISE # 1 HARITHAM ARTS&CRAFTS TRADITIONAL HANDICRAFT THRIKAIPETTA (PO), NELLIMALAM, WAYANAD T-9495240567 2 ABHIRAMI BAMBOO UNIT TRADITIONAL BAMBOO PAZHUPPATHUR P.O PRODUCTS THEKKINKANDY WAYANAD-673592 T-9539595919 3 AISWARYA NUTIMIX FOOD NUTRIMIX NENMENIKKUNNU P.O PROCESSING SULTHAN BETHERY WAYANAD- 673595 T-9747075572 4 SWATHY CURRY POWDER FOOD CURRY POWDER THAVANI PROCESSING NENMENI P.O KOLIYADI SULTHAN BETHERY WAYANAD MOB: 9605974980 5 VALSALYAM NUTRIMIX FOOD CURRY POWDER/ NENMENI.P.O PROCESSING NUTRIMIX MADAKARA SULTHAN BETHERI WAYANAD MOB: 9656051316 [email protected] OM 6 AISWARYA NUTIMIX FOOD NUTRIMIX NOOLPUZHA PANJAYAT PROCESSING NENMENI KUNNU P.O WAYANAD- 673595 MOB: 9744540040 7 JWALA NUTRIMIX FOOD NUTRIMIX KIDAGANAD P O PROCESSING VADAKKANAD SULTHAN BETHERY WAYANAD MOB: 9961137711 8 JEEVANDHARA NEUTRIMIX FOOD NUTRIMIX KIDAGANAD P.O PROCESSING VADAKKANAD WAYANAD MOB: 9744667166 9 JEEVANDHARA NEUTRIMIX FOOD NUTRIMIX KIDAGANAD P.O PROCESSING VADAKKANAD WAYANAD MOB: 9747574461 10 ATHIRA FOOD PRODUCTS FOOD FOOD PRODUCT K21, USHAS PROCESSING KANIYARAM MANANTHAVADY WAYANAD PH: 04935240185 , 9446648051 11 AYSHA CHEMICALS CHEMICALS DETERGENTS POOTHICAUD POOMALA P O WAYANAD PH: 04936222166 12 KOCHIKUNNEL FOOD FOOD BAKERY PRODUCTS KINFRA PARK PROCESSING PRODUCTS CHUNDEL KALPETTA WAYANAD PH: 04936202707 , 9447042677 KOCHIKUNNELFOODPRODUCTS@G MAIL.COM 13 NAS BAGS OTHERS SHOPPER BAGS PUZHAMUDI P.O KALPETTA WAYANAD T-04936204669 , 9567455570 14 KENZ GARMENTS, GARMENTS READYMADE DWARAKA, -

Thalappally Taluk
THALAPPILLY TALUK Kavu Name of Kavu & Custodian Locaon Sy.No. Extent Diety/Pooja GPS Remarks No. Type of Ownership & Address Village (Cents) Details Reading Compound TCR/Tlp Panchayath Wall/ Fence/Pond (1) (2) (3) (4) (5) (6) (7) (8) (9) 1.KANDANASSERY VILLAGE 1 Panthaayil Sarpakavu Chandramathy Thampy Kandaanassery 2.50 Nagaru N 100 CW (Private) Panthaayil House Kandaanassery 1084/3 Bhadrakali 35. 878’ Kandaanassery,Ariyannur Vanadurga E 760 Thriss ‐680102,04885237776 Poojaonceayear 04 .674’ 2 Elathur Sarpakavu Kripavathy Amma Kandaanassery 10.00 Nagaru N 100 CW (Belongs to Elathur Family) Elathur Veedu,Kandaanassery Kandaanassery ‐‐ Once a yaer 36.028’ 04885237716,9447031440 E 760 04.835’ 3 Pallippaa쟼u Sarpakavu P.R.N.Nambeesan Arikanniyur 156/2 1.50 Nagaru N 100 (Belongs to Pallipaa쟼u family) Pallippaa쟼u Veedu, Arikanniyoor Kandaanassery Once a year 36 .65’ Kandaanassery E 760 04. 948’ 4 Nellari Thyvalappil Sarpakavu Rajan. N.S. Kandaanassery 6.00 Mony Nagam N 100 (Family kavu) Nellari Thyvalappil Veedu Kandaanassery ‐‐ Kanni nagam 36.091’ Kandaanassery Brahmarakshas E 760 Thriss – 680102,9496289181 Aayilya 04.801’ 5 Elathur Sarpakavu Rajendran Kandaanassery 1.50 Nagaru N 100 (Family Kavu) Elathur House Kandaanassery ‐‐ Yearly pooja 35.996’ Kandaanassery04885237714 E 760 04.845’ TCR/Tlp Arikanniyoor Sarpakavu A.K. Ramachandran, Arikanniyoor house, E.M.S. Road, Arikanniyoor 107/3 2.50 Maninagam N 100 CW 6 (Private) Arikanniyoor.P.O,9895670343 Kandanasseri Karinagam 36.531’ Seasonal pooja E 760 05.040’ 7 Naduvil Sarpakavu Padmavathy -

Accused Persons Arrested in Thrissur City District from 28.02.2021To06.03.2021
Accused Persons arrested in Thrissur City district from 28.02.2021to06.03.2021 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 135/2021 U/s Sec. 4(2)(e)(j) PANICHAKATH r/w 3(b) of VADAKEK 06-03-2021 20, HOUSE PANANTHA Kerala AD P.R RAJEEV, BAILED BY 1 HASSAN IBRAHIM at 00:00 Male VELIYAMCODE , RA Epidemic (Thrissur SI OF POLICE POLICE Hrs MALAPPURAM Diseases City) Ordinance 2020 PANAMUKU 06-03-2021 181/2021 U/s Pavaratty CHANDRA 31, HOUSE MULLASSER BAILED BY 2 ANPPO at 21:20 118(e) of KP (Thrissur SUJITH R.P N Male THANAVEEDHI Y POLICE Hrs Act City) MULLASSERY THEKKEPPURATH U KUNNAMK 06-03-2021 248/2021 U/s MUHAMME VELAYUDH 53, HOUSE,PERUMAN KUNNAMKU ULAM BAILED BY 3 VENU T V at 21:50 279 IPC & 185 D ABDUL AN Male NU DESAM LAM (Thrissur POLICE Hrs MV ACT NAZIR ,KECHERY City) ,KUNNAMKULAM 133/2021 U/s Sec. 4(2)(e)(j) KATTISSERY r/w 3(b) of VADAKEK 06-03-2021 45, HOUSE KADIKAD PANANTHA Kerala AD P.R RAJEEV, BAILED BY 4 VINAYAN VELAYI at 00:00 Male PUNNAYURKULA RA Epidemic (Thrissur SI OF POLICE POLICE Hrs M Diseases City) Ordinance 2020 132/2021 U/s Sec. 4(2)(e)(j) r/w 3(b) of VADAKEK cheruparambil veed, 06-03-2021 SREEDHAR 20, ALTHARA Kerala AD P.R RAJEEV, BAILED BY 5 VISHNU panthavoor at 00:00 AN Male EAST 2 KM Epidemic (Thrissur SI OF POLICE POLICE vadakkekad Hrs Diseases City) Ordinance 2020 PANNENGATH -
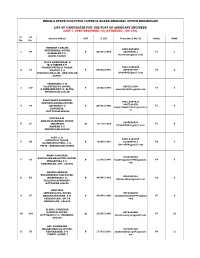
Rank List for the Post of Assistant Engineer
KERALA STATE POLLUTION CONTROL BOARD,REGIONAL OFFICE,ERNAKULAM LIST OF CANDIDATES FOR THE POST OF ASSISTANT ENGINEER (LIST 1 OPEN-REQUIRED 13) (ATTENDED - 69/130) Sl. List Name & Address M/F D.O.B Phone No. & Mail ID Marks RANK No. No. FERZEEN A SALAM 0484-2422456 NETTESSERIL HOUSE 1 84 F 23/08/1992 7034663011 77 1 KUMBALAM P.O. [email protected] KOCHI,682506 DIVYA DAMODARAN B W/O VIBESH T V THOZHUTHUNGAL HOUSE 0484-2680628 2 18 VILANGU P O F 09/02/1988 9048487784 76 2 KIZHAKKAMBALAM , ERNAKULAM [email protected] 683561 ANEESAMOL U M ULLATTUKUDY HOUSE, 8281211994 3 116 F 16/02/1993 73 3 S VAZHAKULAM P O, ALUVA [email protected] ERNAKULAM 683105 SANJU MARY SOBICHEN 0481-2494473 ERAVIKULANGARA HOUSE 9447149158 4 64 DEVAGIRI P O F 24/08/1990 71 4 [email protected] KANGAHZA, m KOTTAYAM 686555 ILLIYAS K.H. KANJIRAPARAMBIL HOUSE 9895656594 5 81 JARAPPADI M 16/12/1992 71 5 [email protected] MANNAM P.O ERNAKULAM 683520 ALET .C.R. 0484-2492765 CHULLIKAL HOUSE, 6 88 F 13/05/1991 8129094717 70 6 ELAMKUNNAPUZHA .P.O. [email protected] VYPIN , ERNAKULAM 682503 BISSY VARGHESE 9746766501 KAPPADAKKAMADATHIL HOUSE, 7 10 F 11/08/1990 [email protected] 69 7 MUDAKUZHA P O, om ERNAKULAM ,PIN - 683546 DHANYA SEKHAR EDACHERIMATTAM HOUSE, 8281332181 8 58 IRUMPANAM P O, F 24/05/1992 68 8 [email protected] THALAYOLAPARAMBU, KOTTAYAM 686605 ANSU MOL ARPPAMALAYIL HOUSE 9072325040 9 97 MADAKKATHANAM .P.O. F 30/04/1990 anusumolvijayan846@gmail 67 9 VAZHAKULAM , MV PA .com ERNAKULAM , 686670 BLESTO CHERIYAN KARIPPAI HOUSE, 8078346889 10 124 M 22/05/1995 67 10 KUTTIKADU P O, THRISSUR, [email protected] 680724 ANU KURIAKOSE MALANCHERUVIL HOUSE, 9495931423 11 35 THOVARAYAR P O F 17/02/1991 [email protected] 66 11 IDUKKI -6855511 om SHOY T S 0487-2342486 THACHANTHRA HOUSE, 12 114 M 20/01/1990 8086865921 65 12 PALISSERY P O PALAKKAL, [email protected] THRISSUR 680021 SUSAN P THOMAS PULIYAMPALLY HOUSE, H NO:8/812 , PLOT NO: 129 , 9895348590 13 13 F 05/05/1990 65 13 SANTHI NAGAR, [email protected] KOOVAPPADAM , ERNAKULAM 9995212787 C.S. -

Hydrogeology and Hydrochemistry of the Aquifer Systems of Kuttanad Area, Kerala: Their Role in Understanding the Evolution of Groundwaters
HYDROGEOLOGY AND HYDROCHEMISTRY OF THE AQUIFER SYSTEMS OF KUTTANAD AREA, KERALA: THEIR ROLE IN UNDERSTANDING THE EVOLUTION OF GROUNDWATERS Thesis submitted to the Cochin University of Science and Technology by Vinayachandran N In partial fulfillment of the requirement for the award of the degree of DOCTOR OF PHILOSOPHY Under the Faculty of Marine Sciences Department of Marine Geology and Geophysics Cochin University of Science and Technology Cochin- 682 016 June 2014 DECLARATION I do hereby declare that the thesis entitled ‘Hydrogeology and hydrochemistry of the aquifer systems of Kuttanad area, Kerala: Their role in understanding the evolution of groundwaters’, being submitted to the Cochin University of Science and Technology, in partial fulfillment of the requirements for award of the degree of Doctor of Philosophy under the faculty of Marine Sciences, is a bona fide record of the work carried out by me in the Department of Marine Geology and Geophysics, Cochin University of Science and Technology, under the supervision of Prof. A.C. Narayana, Professor, Centre for Earth & Space Sciences, University of Hyderabad, Hyderabad (Former Professor , Department of Marine Geology and Geophysics, Cochin University of Science and Technology, Kerala) and that this thesis has not previously formed the basis for the award of any other degree. Vinayachandran N Cochin 21-06-2014 i CERTIFICATE This is to certify that the thesis entitled “Hydrogeology and hydrochemistry of the aquifer systems of Kuttanad area, Kerala: Their role in understanding the evolution of groundwaters” is an authentic record of research work carried out by Mr. Vinayachandran N, under my supervision and guidance at the Department of Marine Geology and Geophysics, Cochin University of Science and Technology, in the Faculty of Marine Sciences, in partial fulfillment of the requirements for the degree of Doctor of Philosophy and no part thereof has been presented for the award of any degree in any University/ Institute. -

Judicial Department
© Regn. No. KERBIL/2012/45073 tIcf k¿°m¿ dated 5-9-2012 with RNI Government of Kerala Reg. No. KL/TV(N)/634/2015-17 2016 tIcf Kkddv KERALA GAZETTE B[nImcnIambn {]kn≤s∏SpØp∂Xv PUBLISHED BY AUTHORITY 2016 Pq¨ 14 Xncph\¥]pcw, hmeyw 5 14th June 2016 \º¿ sNmΔ 1191 CShw 31 31st Idavam 1191 24 Vol. V } Thiruvananthapuram, No. } 1938 tPyjvTw 24 Tuesday 24th Jyaishta 1938 PART III Judicial Department THE HIGH COURT OF KERALA Office of the Chief Judicial Magistrate, NOTIFICATION Ernakulam No. D1(A)81887/2007/D1. 18th May 2016. NOTIFICATIONS Sub:—Rule Committee constituted under Section (1) 123 of the Code of Civil Procedure, 1908— Appointment of new Member—Reg. No. A3-3509/2016. 11th May 2016. Ref:—High Court Notifications of even number In exercise of the powers conferred under section dated 7-10-2014 & 18-4-2015. 14 (1) of Code of Criminal Procedure 1973 (Central Act 2 In exercise of the powers conferred by section 123 of the Code of Civil Procedure, 1908, the High Court of of 1974) and Order of the Hon’ble High Court of Kerala Kerala hereby reconstitute the Rule Committee by bearing No. D7A (2)-6788/2015 dated 22-4-2016, the Chief including Honourable Mr. Justice A. Hariprasad as a Judicial Magistrate, Ernakulam hereby define the areas Member of the Rule Committee in the place of the Honourable Mr. Justice P. Bhavadasan, who has retired specified in Column No. (4) of the Schedule-I to the from service. local limits of the area within which the Court of the Judicial Magistrate of the First Class mentioned in Column By order, N. -

Accused Persons Arrested in Thrissur City District from 21.06.2020To27.06.2020
Accused Persons arrested in Thrissur City district from 21.06.2020to27.06.2020 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 3091/2020 U/s 269, 290 IPC & 118(e) of KP Act & Thrissur Parambhil 27-06-2020 Sec. 4(2)(d) 29, East BAILED BY 1 Sovin Sojan tharakkan house, Jose junction at 23:10 r/w 5 of Bibin c v Male (Thrissur POLICE ollur desam, ollur Hrs Kerala City) Epidemic Diseases Ordinance 2020 3090/2020 U/s 269, 290 IPC & 118(e) of KP Act & Thrissur 27-06-2020 Sec. 4(2)(d) 18, Panikkavettil house, East BAILED BY 2 Asab Latheef Jose junction at 22:50 r/w 5 of Bibin c v Male kalathode (Thrissur POLICE Hrs Kerala City) Epidemic Diseases Ordinance 2020 3089/2020 U/s 269, 290 IPC & 118(e) of KP Act & VIMALALAYAM Thrissur NIRMAL 27-06-2020 Sec. 4(2)(f) SREEKUMA 23, HOUSE,NETTISER JOSE East BAILED BY 3 SREEKUMA at 22:40 r/w 5 of BIBIN C V R Male RY,MUKATTUKKA JUNCTION (Thrissur POLICE R Hrs Kerala RA City) Epidemic Diseases Ordinance 2020 3088/2020 U/s 269, 290 IPC & 118(e) of KP Act & Thrissur ADATT 27-06-2020 Sec. 4(2)(f) SOORYAVA 28, JOSE East BAILED BY 4 SOMAN HOUSE,ADATT,TH at 22:25 r/w 5 of BIBIN C V RTHAN Male JUNCTION (Thrissur POLICE RISSUR Hrs Kerala City) Epidemic Diseases Ordinance 2020 386/2020 U/s 118(e) of KP THEKKANATHU( Act & Sec.