A THESIS Entitled the INFLUENCE of ENVIRONMENT on REGIONAL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
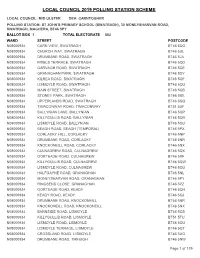
Local Council 2019 Polling Station Scheme
LOCAL COUNCIL 2019 POLLING STATION SCHEME LOCAL COUNCIL: MID ULSTER DEA: CARNTOGHER POLLING STATION: ST JOHN'S PRIMARY SCHOOL (SWATRAGH), 30 MONEYSHARVAN ROAD, SWATRAGH, MAGHERA, BT46 5PY BALLOT BOX 1 TOTAL ELECTORATE 882 WARD STREET POSTCODE N08000934CARN VIEW, SWATRAGH BT46 5QG N08000934CHURCH WAY, SWATRAGH BT46 5UL N08000934DRUMBANE ROAD, SWATRAGH BT46 5JA N08000934FRIELS TERRACE, SWATRAGH BT46 5QD N08000934GARVAGH ROAD, SWATRAGH BT46 5QE N08000934GRANAGHAN PARK, SWATRAGH BT46 5DY N08000934KILREA ROAD, SWATRAGH BT46 5QF N08000934LISMOYLE ROAD, SWATRAGH BT46 5QU N08000934MAIN STREET, SWATRAGH BT46 5QB N08000934STONEY PARK, SWATRAGH BT46 5BE N08000934UPPERLANDS ROAD, SWATRAGH BT46 5QQ N08000934TIMACONWAY ROAD, TIMACONWAY BT51 5UF N08000934BALLYNIAN LANE, BALLYNIAN BT46 5QP N08000934KILLYGULLIB ROAD, BALLYNIAN BT46 5QR N08000934LISMOYLE ROAD, BALLYNIAN BT46 5QU N08000934BEAGH ROAD, BEAGH (TEMPORAL) BT46 5PX N08000934CORLACKY HILL, CORLACKY BT46 5NP N08000934DRUMBANE ROAD, CORLACKY BT46 5NR N08000934KNOCKONEILL ROAD, CORLACKY BT46 5NX N08000934CULNAGREW ROAD, CULNAGREW BT46 5QX N08000934GORTEADE ROAD, CULNAGREW BT46 5RF N08000934KILLYGULLIB ROAD, CULNAGREW BT46 5QW N08000934LISMOYLE ROAD, CULNAGREW BT46 5QU N08000934HALFGAYNE ROAD, GRANAGHAN BT46 5NL N08000934MONEYSHARVAN ROAD, GRANAGHAN BT46 5PY N08000934RINGSEND CLOSE, GRANAGHAN BT46 5PZ N08000934GORTEADE ROAD, KEADY BT46 5QH N08000934KEADY ROAD, KEADY BT46 5QJ N08000934DRUMBANE ROAD, KNOCKONEILL BT46 5NR N08000934KNOCKONEILL ROAD, KNOCKONEILL BT46 5NX N08000934BARNSIDE ROAD, LISMOYLE -

Binevenagh Binevenagh Make to Combine That Features Distinctive
National Trust acquired the property in 1976. in property the acquired Trust National Magilligan Point ©Tourism NI ©Tourism Point Magilligan rail journeys in the world”. the in journeys rail farmer, Isaac Hezlett, in 1761. His family lived there until the the until there lived family His 1761. in Hezlett, Isaac farmer, Londonderry and Coleraine as “one of the most beautiful beautiful most the of “one as Coleraine and Londonderry the rector of Dunboe and was taken over by a Presbyterian Presbyterian a by over taken was and Dunboe of rector the writer Michael Palin described the train journey between between journey train the described Palin Michael writer ‘crucks’. The cottage was probably built as a parsonage for for parsonage a as built probably was cottage The ‘crucks’. Ireland, measuring 610 and 280 metres respectively. Travel Travel respectively. metres 280 and 610 measuring Ireland, walls hide a fascinating early frame of curved timbers called called timbers curved of frame early fascinating a hide walls and Downhill – they are still the longest railway tunnels in in tunnels railway longest the still are they – Downhill and Ireland’s oldest surviving thatched cottage, its roughcast roughcast its cottage, thatched surviving oldest Ireland’s through two headlands on the route between Castlerock Castlerock between route the on headlands two through cottage dating from around 1691. Not only is it Northern Northern it is only Not 1691. around from dating cottage major engineering achievement, requiring tunnels to be cut cut be to tunnels requiring achievement, engineering major Hezlett House outside Castlerock, is a beautiful thatched thatched beautiful a is Castlerock, outside House Hezlett Company opened a line between these two towns. -

Mineral Exploration Potential in the North of Ireland
8. Critical metals for high- technology applications: mineral exploration potential in the north of Ireland Paul Lusty1 How to cite this chapter: There is global concern about the availability of ‘critical’ metals: those of growing eco- Lusty, P.A.J., 2016 ‘Critical nomic importance but vulnerable to supply shortage. Production from domestic resources metals for high-technology could contribute to security of supply. However, we have little information on how critical applications: mineral exploration potential in the metals are concentrated in the Earth’s crust and the resources that exist in the British Isles. north of Ireland’ in M.E. Ireland’s diverse geology provides many geological environments in which critical metals Young (ed.), Unearthed: impacts of the Tellus surveys of may be enriched. This review considers mineral exploration potential for selected ‘criti- the north of Ireland. Dublin. cal’ metals identified by the European Commission and others considered important for Royal Irish Academy. high-technology applications. Known mineral deposits and the Tellus and Tellus Border DOI:10.3318/ geochemistry suggest that the north of Ireland is prospective for some of these metals and 978-1-908996-88-6.ch8 warrants further investigation. Extraction of these metals as by-products could facilitate the development of otherwise sub-economic ore bodies in Ireland, thus supporting eco- nomic growth. Introduction Global concerns are growing over the long-term availability of secure and adequate sup- plies of the minerals and metals needed by society. Of particular concern are the ‘critical’ raw materials, so called because of their growing economic importance and high risk of supply shortage. -

Patriots, Pioneers and Presidents Trail to Discover His Family to America in 1819, Settling in Cincinnati
25 PLACES TO VISIT TO PLACES 25 MAP TRAIL POCKET including James Logan plaque, High Street, Lurgan FROM ULSTER ULSTER-SCOTS AND THE DECLARATION THE WAR OF 1 TO AMERICA 2 COLONIAL AMERICA 3 OF INDEPENDENCE 4 INDEPENDENCE ULSTER-SCOTS, The Ulster-Scots have always been a transatlantic people. Our first attempted Ulster-Scots played key roles in the settlement, The Ulster-Scots/Scotch-Irish contribution to the Patriot cause in the events The Ulster-Scots/Scotch-Irish played important roles in the military aspects of emigration was in 1636 when Eagle Wing sailed from Groomsport for New England administration and defence of Colonial America. leading up to and including the American War of Independence was immense. the War of Independence. General Richard Montgomery was the descendant of SCOTCH-IRISH but was forced back by bad weather. It was 1718 when over 100 families from the Probably born in County Donegal, Rev. Charles Cummings (1732–1812), a a Scottish cleric who moved to County Donegal in the 1600s. At a later stage the AND SCOTS-IRISH Bann and Foyle river valleys successfully reached New England in what can be James Logan (1674-1751) of Lurgan, County Armagh, worked closely with the Penn family in the Presbyterian minister in south-western Virginia, is believed to have drafted the family acquired an estate at Convoy in this county. Montgomery fought for the regarded as the first organised migration to bring families to the New World. development of Pennsylvania, encouraging many Ulster families, whom he believed well suited to frontier Fincastle Resolutions of January 1775, which have been described as the first Revolutionaries and was killed at the Battle of Quebec in 1775. -

Northern Ireland Information for H4010
European Community Directive on the Conservation of Natural Habitats and of Wild Fauna and Flora (92/43/EEC) Fourth Report by the United Kingdom under Article 17 on the implementation of the Directive from January 2013 to December 2018 Supporting documentation for the conservation status assessment for the habitat: H4010 ‐ Northern Atlantic wet heaths with Erica tetralix NORTHERN IRELAND IMPORTANT NOTE ‐ PLEASE READ • The information in this document is a country‐level contribution to the UK Reporton the conservation status of this habitat, submitted to the European Commission aspart of the 2019 UK Reporting under Article 17 of the EU Habitats Directive. • The 2019 Article 17 UK Approach document provides details on how this supporting information was used to produce the UK Report. • The UK Report on the conservation status of this habitat is provided in a separate doc‐ ument. • The reporting fields and options used are aligned to those set out in the European Com‐ mission guidance. • Explanatory notes (where provided) by the country are included at the end. These pro‐ vide an audit trail of relevant supporting information. • Some of the reporting fields have been left blank because either: (i) there was insuffi‐ cient information to complete the field; (ii) completion of the field was not obligatory; and/or (iii) the field was only relevant at UK‐level (sections 10 Future prospects and11 Conclusions). • For technical reasons, the country‐level future trends for Range, Area covered by habitat and Structure and functions are only available in a separate spreadsheet that contains all the country‐level supporting information. • The country‐level reporting information for all habitats and species is also available in spreadsheet format. -

Co. Londonderry – Historical Background Paper the Plantation
Co. Londonderry – Historical Background Paper The Plantation of Ulster and the creation of the county of Londonderry On the 28th January 1610 articles of agreement were signed between the City of London and James I, king of England and Scotland, for the colonisation of an area in the province of Ulster which was to become the county of Londonderry. This agreement modified the original plan for the Plantation of Ulster which had been drawn up in 1609. The area now to be allocated to the City of London included the then county of Coleraine,1 the barony of Loughinsholin in the then county of Tyrone, the existing town at Derry2 with adjacent land in county Donegal, and a portion of land on the county Antrim side of the Bann surrounding the existing town at Coleraine. The Londoners did not receive their formal grant from the Crown until 1613 when the new county was given the name Londonderry and the historic site at Derry was also renamed Londonderry – a name that is still causing controversy today.3 The baronies within the new county were: 1. Tirkeeran, an area to the east of the Foyle river which included the Faughan valley. 2. Keenaght, an area which included the valley of the river Roe and the lowlands at its mouth along Lough Foyle, including Magilligan. 3. Coleraine, an area which included the western side of the lower Bann valley as far west as Dunboe and Ringsend and stretching southwards from the north coast through Macosquin, Aghadowey, and Garvagh to near Kilrea. 4. Loughinsholin, formerly an area in county Tyrone, situated between the Sperrin mountains in the west and the river Bann and Lough Neagh on the east, and stretching southwards from around Kilrea through Maghera, Magherafelt and Moneymore to the river Ballinderry. -

Outdoor Recreation Action Plan for the Sperrins (ORNI on Behalf of Sportni, 2013)
Mid Ulster District Council Outdoor Recreation Strategic Plan Prepared by Outdoor Recreation NI on behalf of Mid Ulster District Council October 2019 CONTENTS CONTENTS ...................................................................................................................................................................................... 1 TABLE OF FIGURES .................................................................................................................................................... 6 TABLE OF TABLES ...................................................................................................................................................... 5 ACRONYMS ........................................................................................................................................................ 6 FOREWORD ........................................................................................................................................................ 7 EXECUTIVE SUMMARY ...................................................................................................................................... 8 1.1 Introduction .......................................................................................................................................12 1.2 Aim ....................................................................................................................................................12 1.3 Objectives .........................................................................................................................................13 -

EONI-REP-223 - Streets - Streets Allocated to a Polling Station by Area Local Council Elections: 02/05/2019
EONI-REP-223 - Streets - Streets allocated to a Polling Station by Area Local Council Elections: 02/05/2019 LOCAL COUNCIL: MID ULSTER DEA: CARNTOGHER ST JOHN'S PRIMARY SCHOOL (SWATRAGH), 30 MONEYSHARVAN ROAD, SWATRAGH, MAGHERA, BT46 5PY BALLOT BOX 1/CN TOTAL ELECTORATE 880 WARD STREET POSTCODE N08000934 CARN VIEW, SWATRAGH, MAGHERA BT46 5QG N08000934 CHURCH WAY, SWATRAGH, MAGHERA BT46 5UL N08000934 DRUMBANE ROAD, SWATRAGH, MAGHERA BT46 5JA N08000934 FRIELS TERRACE, SWATRAGH, MAGHERA BT46 5QD N08000934 GARVAGH ROAD, SWATRAGH, MAGHERA BT46 5QE N08000934 GRANAGHAN PARK, SWATRAGH, MAGHERA BT46 5DY N08000934 KILREA ROAD, SWATRAGH, MAGHERA BT46 5QF N08000934 LISMOYLE ROAD, SWATRAGH, MAGHERA BT46 5QU N08000934 MAIN STREET, SWATRAGH, MAGHERA BT46 5QB N08000934 STONEY PARK, SWATRAGH, MAGHERA BT46 5BE N08000934 UPPERLANDS ROAD, SWATRAGH, MAGHERA BT46 5QQ N08000934 TIMACONWAY ROAD, TIMACONWAY, KILREA BT51 5UF N08000934 BALLYNIAN LANE, BALLYNIAN, SWATRAGH BT46 5QP N08000934 KILLYGULLIB ROAD, BALLYNIAN, SWATRAGH BT46 5QR N08000934 LISMOYLE ROAD, BALLYNIAN, SWATRAGH BT46 5QU N08000934 BEAGH ROAD, BEAGH (TEMPORAL), SWATRAGH BT46 5PX N08000934 CORLACKY HILL, CORLACKY, SWATRAGH BT46 5NP N08000934 DRUMBANE ROAD, CORLACKY, SWATRAGH BT46 5NR N08000934 KNOCKONEILL ROAD, CORLACKY, SWATRAGH BT46 5NX N08000934 CULNAGREW ROAD, CULNAGREW, SWATRAGH BT46 5QX N08000934 GORTEADE ROAD, CULNAGREW, SWATRAGH BT46 5RF N08000934 KILLYGULLIB ROAD, CULNAGREW, SWATRAGH BT46 5QW N08000934 LISMOYLE ROAD, CULNAGREW, SWATRAGH BT46 5QU N08000934 HALFGAYNE ROAD, GRANAGHAN, SWATRAGH -

Landscape Assessment Position Paper
Mid Ulster Position Paper – Landscape Assessment September 2015 Prepared by Mid Ulster Environment and Conservation Team. Landscape Assessment of Mid Ulster Council Purpose: To provide members with a Landscape Assessment for Mid Ulster Council to highlight those areas most vulnerable to change within the district. Content: The paper provides information on:- (i) The Northern Ireland Landscape Character Assessment for Mid Ulster District and its key findings; (ii) In addition to identifying the key characteristics of each landscape character area, the report also assesses the principal forces for change and issues influencing landscape condition and sensitivity. (iii) This paper forms part of the Countryside Assessment for Mid Ulster and is to be read in conjunction with Environmental Assets Paper, Strategic Settlement Appraisal Paper and Development Pressure Analysis Paper. Recommendation: That the Planning Committee notes the contents of this paper in relation to our districts diverse landscape character and its interaction with the planning function. 2 1.0 Introduction 1.1 Mid Ulster comprises a diverse mix of landscapes including mountains and moorlands, bog lands, drumlins, lowlands and important river valleys. Recognition of landscapes of national importance is given through the designation of Areas of Outstanding Natural Beauty (AONB). A significant part of the Sperrin AONB (designated 2008) lies within the Mid Ulster District. The purpose of the AONB designation is to protect and conserve the scenic qualities of the area and promote their enjoyment. This is supported by two designated Areas of High Scenic Value within Mid Ulster District, namely West Lough Neagh Shores and Slieve Gallion Slopes. 1.2 All of the NI landscape has been classified by the Northern Ireland Landscape Character Assessment 2000 (NILCA 2000), which was compiled by the Northern Ireland Environment Agency. -

Tel. 028 8556 7723
Register early Completed application forms with payment (cheques made to reserve your chosen kit size. payable to Tyrone GAA), can be returned to your local primary school or posted to the address below: Garvaghey Centre, 230 Redergan Road, Dungannon, Co. Tyrone BT70 2EH. Tel. 028 8556 7723 “5 -13 year olds can attend Camps” Application forms available from www.tyronegaa.ie There will be a late registration fee of £5 per child for registrations received after the 27th June. Garvaghey Centre, Please tick camp attending 230 Redergan Road, Dungannon, Co. Tyrone BT70 2EH. Hurling/Camogie Football Multi-sport Tel. 028 8556 7723 Name/s ........................................................................................... Application forms available from ........................................................................................................ Age/s .............................................................................................. www.tyronegaa.ie 2014 School/s .......................................................................................... ........................................................................................................ Home Address ................................................................................ ........................................................................................................ ........................................................................................................ Name of Parent / Guardian............................................................ -

Foyle Heritage Audit NI Core Document
Table of Contents Executive Summary i 1 Introduction ..................................................................................................1 1.1 Purpose of Study ................................................................................................... 1 1.2 Objectives of the Audit ......................................................................................... 2 1.3 Project Team ......................................................................................................... 3 1.4 Study Area ............................................................................................................. 5 1.5 Divisions ................................................................................................................ 6 2 Audit Methodology .......................................................................................8 2.1 Identification of Sources ....................................................................................... 8 2.2 Pilot Study Area..................................................................................................... 9 2.3 Selection & Organisation of Data .......................................................................... 9 2.4 Asset Data Sheets ............................................................................................... 11 2.5 Consultation & Establishment of Significance .................................................... 11 2.6 Public Presentation ............................................................................................ -
Exploring the History & Heritage of Tyrone and the Sperrins
Exploring the History & Heritage of Tyrone and The Sperrins Millennium Sculpture Strabane Canal Artigarvan & Leckpatrick Moor Lough Lough Ash Plumbridge & The Glenelly Valley The Wilson Ancestral Home Sion Mills Castlederg Killeter Village Ardstraw Graveyard Stewart Castle Harry Avery’s Castle Patrick Street Graveyard, Strabane pPB-1 Heritage Trail Time stands still; time marches on. It’s everywhere you look. In our majestic mountains and rivers, our quiet forests and rolling fields, in our lively towns and scenic villages: history is here, alive and well. Some of that history is ancient and mysterious, its archaeology shaping our landscape, even the very tales we tell ourselves. But there are other, more recent histories too – of industry and innovation; of fascinating social change and of a vibrant, living culture. Get the full Local visitor App experience: information: Here then is the story of Tyrone and the Sperrins - Download it to your iphone The Alley Artsan and extraordinary journey through many worlds, from or android smartphone Conference Centre 1A Railway Sdistanttreet, Str pre-historyabane all the way to the present day. and discover even more Co. Tyrone, BT82 8EF about the History & Heritage It’s a magical, unforgettable experience. of Tyrone and The Sperrins. Email: [email protected] Web:www.discovertyroneandsperrins.com Tel: (028) 71Join38 4444 us and discover that as time marches on, time also stands still… p2-3 x the sites The sites are categorised 1 Millennium Sculpture 6 by heritage type as below 2 Strabane Canal 8