Securing Trust in the Global COVID-19 Supply Chain Securing Trust in the Global COVID-19 Supply Chain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KDHE News Release
News Room - 2012 - News Release A to Z Topic Listing KDHE News Release For Immediate Release KDHE Office of Communications January 3, 2012 [email protected], 785-296-0461 KDHE Promotes Cancer Screenings and HPV Vaccination During Cervical Health Awareness Month TOPEKA – In recognition of Cervical Health Awareness Month, the Kansas Department of Health and Environment (KDHE) encourages women to schedule their annual well-woman checkups. According to a 2010 Kansas Behavioral Risk Factor Surveillance System (BRFSS) report, approximately 17 percent of Kansas women aged 18 and older did not have a Pap test within the past three years. "Pap tests decrease the risk of developing cervical cancer by detecting precancerous cells which, when found early, are highly treatable," said Robert Moser, M.D., KDHE Secretary and State Health Officer. “Women should have their first screening Pap test at age 21, or within three years of becoming sexually active if younger." Although cervical cancer was once the leading cancer killer of women, the number of cases has declined 75 percent in the past 50 years, largely because of the widely available and reliable Pap test. Even so, an estimated 12,000 women in the United States are diagnosed with cervical cancer each year. In 2007, 4,021 women died from cervical cancer in the United States. In 2008, 76 Kansas cases were diagnosed, with 24 deaths due to cervical cancer in 2010. Most cervical cancer cases are caused by infection with human papillomavirus (HPV), a common sexually transmitted disease. HPV infection can also cause a number of other health problems for both men and women. -

Gavi's Vaccine Investment Strategy
Gavi’s Vaccine Investment Strategy Deepali Patel THIRD WHO CONSULTATION ON GLOBAL ACTION PLAN FOR INFLUENZA VACCINES (GAP III) Geneva, Switzerland, 15-16 November 2016 www.gavi.org Vaccine Investment Strategy (VIS) Evidence-based approach to identifying new vaccine priorities for Gavi support Strategic investment Conducted every 5 years decision-making (rather than first-come- first-serve) Transparent methodology Consultations and Predictability of Gavi independent expert advice programmes for long- term planning by Analytical review of governments, industry evidence and modelling and donors 2 VIS is aligned with Gavi’s strategic cycle and replenishment 2011-2015 Strategic 2016-2020 Strategic 2021-2025 period period 2008 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 RTS,S pilot funding decision VIS #1 VIS #2 VIS #3 MenA, YF mass campaigns, JE, HPV Cholera stockpile, Mid 2017 : vaccine ‘long list’ Rubella, Rabies/cholera studies, Oct 2017 : methodology Typhoid Malaria – deferred Jun 2018 : vaccine shortlist conjugate Dec 2018 : investment decisions 3 VIS process Develop Collect data Develop in-depth methodology and Apply decision investment decision framework for cases for framework with comparative shortlisted evaluation analysis vaccines criteria Phase I Narrow long list Phase II Recommend for Identify long list to higher priority Gavi Board of vaccines vaccines approval of selected vaccines Stakeholder consultations and independent expert review 4 Evaluation criteria (VIS #2 – 2013) Additional Health Implementation -

Yellow Fever 2016
Resident / Humanitarian Coordinator Report on the use of CERF funds RESIDENT / HUMANITARIAN COORDINATOR REPORT ON THE USE OF CERF FUNDS ANGOLA RAPID RESPONSE YELLOW FEVER 2016 RESIDENT/HUMANITARIAN COORDINATOR Pier Paolo Balladelli REPORTING PROCESS AND CONSULTATION SUMMARY a. Please indicate when the After Action Review (AAR) was conducted and who participated. Review agreed on 02/09/2016 and 07/09/2016. b. Please confirm that the Resident Coordinator and/or Humanitarian Coordinator (RC/HC) Report was discussed in the Humanitarian and/or UN Country Team and by cluster/sector coordinators as outlined in the guidelines. YES NO c. Was the final version of the RC/HC Report shared for review with in-country stakeholders as recommended in the guidelines (i.e. the CERF recipient agencies and their implementing partners, cluster/sector coordinators and members and relevant government counterparts)? YES NO Final version shared with UNICEF and UNDP, although this initiative was mainly implemented by WHO 2 I. HUMANITARIAN CONTEXT TABLE 1: EMERGENCY ALLOCATION OVERVIEW (US$) Total amount required for the humanitarian response: Source Amount CERF 3,000,000 Breakdown of total response COUNTRY-BASED POOL FUND (if applicable) 4,508,559 funding received by source OTHER (bilateral/multilateral) TOTAL 10,473,618 *The total amount does not match because this was considered an underfunded emergency. TABLE 2: CERF EMERGENCY FUNDING BY ALLOCATION AND PROJECT (US$) Allocation 1 – date of official submission: 06/04/2016- 05/10/2016 Agency Project code Cluster/Sector -

Hanna Nohynek @Hnohynek
Biosketch Hanna Nohynek @hnohynek Hanna Nohynek is Chief Physician and Deputy Head of the Infectious Diseases Control and Vaccines Unit of the Department of Health Security at the Finnish Institute for Health and Welfare. She serves as secretary of the Finnish NITAG (KRAR), and leads the subgroup on Strategic development of the influenza vaccination programme and the subgroup on the SARS-CoV-2 vaccination strategy. She practices clinical medicine at a travel health clinic in Aava, Helsinki. She was instrumental in designing the first THL (KTL) health advisory for refugees and asylum seekers in Finland, studying the narcolepsy signal post pandemic vaccination, designing the introduction of the HPV vaccine to the national immunization programme, and the introduction of the live attenuated influenza vaccine for children. Her present research interests are register-based vaccine impact studies, evidence based policy/decision making, vaccine safety, hesitancy, SARS-CoV-2, RSV, influenza and pneumococcus. She coordinates the work packages on field studies and communication for IMI DRIVE on brand specific influenza vaccine effectiveness (www.drive-eu.org). She has authored more than 130 original articles (including the first scientific report on the association between pandemic influenza vaccination and narcolepsy), and she teaches, giving over 30 invited lectures annually and guiding elective, graduate and PhD students (presently Raija Auvinen and Idil Hussein). She belongs to the external faculty of the University of Tampere MSc course on Global Health. She has served on expert committees evaluating HBV, PCV and rota virus vaccines in Finland, and as an advisor to the EU, IMI, IVI, WHO, GAVI, SIDA/SRC, and the Finnish MOFA. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
Storage Best Practices for Frozen Vaccines-Fahrenheit
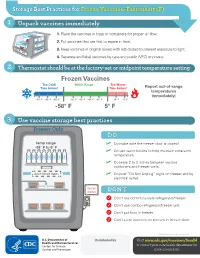
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

CV Seth Berkly2.Indd
Dr Seth Berkley Chief Executive Officer of the GAVI Alliance As a physician, epidemiologist and leading advocate for vaccines and immunisation, Dr Seth Berkley brings many years of experience and knowledge from the fields of public health, development and vaccines to the work of the Alliance. Seth joined the GAVI Alliance in August 2011 as it launched its five year strategy to immunise a quarter of a billion children in the developing world with life-saving vaccines by 2015. “I am impressed by GAVI’s life-saving work and have great admiration for its successful track record,” said Dr Berkley. “I am therefore honoured and excited to have been chosen to lead the GAVI Alliance as it works to deliver on an ambitious new strategy.” Prior to joining the GAVI Alliance, Seth was the founder, president and CEO for 15 years of the International AIDS Vaccine Initiative ( IAVI ), the first vaccine product development public-private sector partnership. Under his leadership, IAVI implemented a global advocacy programme that assured that vaccines received prominent attention in the media and in political forums such as the G 8, EU and the UN. He also oversaw the creation of a virtual vaccine product development effort involving industry, academia, and developing country scientists. Prior to founding IAVI, Seth served as associate director in the Health Sciences Division at The Rockefeller Foundation. He has also worked for the Center for Infectious Diseases of the U. S. Centers for Disease Control and Prevention ( CDC ), the Massachusetts Department of Public Health and for the Carter Center where he served as an epidemiologist at the Ministry of Health in Uganda. -

UNEP Mercury Treaty Protects Access to Thiomersal-Containing Vaccines
UNEP mercury treaty protects access to thiomersal-containing vaccines United Nations Environment Program has developed a treaty on mercury in an effort to protect human health and the environment by limiting mercury releases. In the course of the negotiations, a proposal was made to restrict vaccines that contain the preservative thiomersal under a section of the treaty that prohibits trade of mercury-added products. The implications of restricting thiomersal, an ethyl mercury-containing preservative, would be significant. According to SAGE, “Thiomersal-containing vaccines [are] safe, essential, and irreplaceable components of immunization programs, especially in developing countries, and…removal of these products would disproportionately jeopardize the health and lives of the most disadvantaged children worldwide.” The treaty annex that describes prohibited products specifically excludes “vaccines containing thiomersal as preservatives” under a short list of products the authors intended to emphasize were to be protected. Protecting access to vaccines came as the result of a strong partnership between WHO, UNICEF, GAVI, and civil society advocates and experts around the world to educate country delegates, who predominantly came from ministries of environment. This was also a wonderful partnership with animal health experts, who similarly rely on thiomersal for veterinary vaccines. By facilitating communication between ministries of health and ministries of environment, strong statements are made by delegates about the essential role of thiomersal-containing vaccines in protecting human health. PATH will be collecting and disseminating additional information about how the community came together around this issue and lessons learned in the coming months. . -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

Cheat Sheet: COVID-19 Vaccine Pipeline
Cheat Sheet: COVID-19 vaccine pipeline Primary sponsor(s) Description Platform Funders Status Considerations Read more Pfizer / BioNTech Comirnaty mRNA Pfizer ($500M) Ph. I/II ongoing: 456/Germany Efficacy: Interim analysis shows that the candidate was safe and well-tolerated with New York Times mRNA that encodes for USG ($1.9M) Ph. II planned: 960/China an efficacy rate of 95%. SARS-CoV-2 spike protein. Warp Speed Finalist Ph. II/III ongoing: 44K US +5 Manufacturing/delivery: mRNA vaccines are relatively easy to scale and Authorization: EUA in EU, US, +9; WHO manufacture. Emergency Validation Platform history: No previous mRNA vaccines licensed for use. Approval: Bahrain, New Zealand, Saudi Arabia, Switzerland Moderna mRNA-1273 mRNA USG ($2.48B) Ph. I ongoing: 155/US Efficacy: Interim analysis shows that the candidate was safe and well-tolerated Moderna Synthetic messenger RNA CEPI/GAVI (Undisclosed) Ph. II/III ongoing: 3000 (12 to 17 years)/ US with an efficacy rate of 94.5%. Statement that encodes for SARS- Warp Speed Finalist Ph. III ongoing: 30,000/US Manufacturing/delivery: mRNA vaccines are relatively easy to scale and CoV-2 spike protein. COVAX Portfolio Authorization: EUA in Canada, EU, manufacture (potential for 1B doses by 2022); likely to require two doses, but a AVAC Israel, US third may be necessary. Webinar Approval: Switzerland Platform history: No previous mRNA vaccines licensed for use. U. of Oxford AZD1222 Viral USG ($1.2B) Ph. II ongoing: 300 vols (6-17 years)/ UK Efficacy: Ph. III interim analysis shows vaccine was safe and well-tolerated, efficacy Science AstraZeneca Chimpanzee Adeno vector vector CEPI/GAVI ($750M) Ph. -

Yellow Fever Vaccine
Yellow Fever Vaccine: Current Supply Outlook UNICEF Supply Division May 2016 0 Yellow Fever Vaccine - Current Supply Outlook May 2016 This update provides revised information on yellow fever vaccine supply availability and increased demand. Despite slight improvements in availability and the return of two manufacturers from temporary suspension, a constrained yellow fever vaccine market will persist through 2017, exacerbated by current emergency outbreak response requirements. 1. Summary Yellow fever vaccine (YFV) supply through UNICEF remains constrained due to limited production capacity. Despite the return of two manufacturers from temporary suspension, the high demand currently generated from the yellow fever (YF) outbreak in Angola, in addition to potential increased outbreak response requirements in other geographic regions, outweigh supply. The demand in response to the current YF outbreak in Angola could negatively impact the supply availability for some routine immunization programme activities. UNICEF anticipates a constrained global production capacity to persist through 2017. UNICEF has long-term arrangements (LTAs) with four YFV suppliers to cover emergency stockpile, routine immunization, and preventative campaign requirements. During 2015, UNICEF increased total aggregate awards to suppliers to reach approximately 98 million doses for 2016- 2017. However, whereas supply can meet emergency stockpile and routine requirements, it is insufficient to meet all preventive campaign demands, which increased the total demand through UNICEF to 109 million doses. The weighted average price (WAP) per dose for YFV increased 7% a year on average since 2001, from US$ 0.39 to reach US$ 1.04 in 2015. Given the continued supply constraints, UNICEF anticipates a YFV WAP per dose of US$1.10 in the near-term. -

An Overview of COVID Vaccine Clinical Trial Results & Some Challenges
An overview of COVID vaccine clinical trial results & some challenges DCVMN Webinar December 8th, 2020 Access to COVID-19 tools ACCESSACCESS TO TOCOVID-19 COVID-19 TOOLS TOOLS (ACT) (ACT) ACCELERATOR ACCELERATOR (ACT) accelerator A GlobalA GlobalCollaboration Collaboration to Accelerate tothe AccelerateDevelopment, the Production Development, and Equitable Production Access to New and Equitable AccessCOVID-19 to New diagnostics, COVID-19 therapeutics diagnostics, and vaccines therapeutics and vaccines VACCINES DIAGNOSTICS THERAPEUTICS (COVAX) Development & Manufacturing Led by CEPI, with industry Procurement and delivery at scale Led by Gavi Policy and allocation Led by WHO Key players SOURCE: (ACT) ACCELERATOR Commitment and Call to Action 24th April 2020 ACT-A / COVAX governance COVAX COORDINATION MEETING CEPI Board Co-Chair: Jane Halton Co-Chair: Dr. Ngozi Gavi Board Workstream leads + DCVMN and IFPMA-selected Reps As needed – R&D&M Chair; COVAX IPG Chair Development & Manufacturing Procurement and delivery Policy and allocation (COVAX) at scale Led by (with industry) Led by Led by R&D&M Investment Committee COVAX Independent Product Group Technical Review Group Portfolio Group Vaccine Teams SWAT teams RAG 3 COVAX SWAT teams are being set up as a joint platform to accelerate COVID- 19 Vaccine development and manufacturing by addressing common challenges together Timely and targeted Multilateral Knowledge-based Resource-efficient Addresses specific cross- Establishes a dialogue Identifies and collates Coordinates between developer technical and global joint effort most relevant materials different organizations/ challenges as they are across different COVID-19 and insights across the initiatives to limit raised and/or identified vaccines organizations broader COVID-19 duplications and ensure on an ongoing basis (incl.